Reproduced and modified with permission. Lee LA, Caplan RA, Stephens LS, Posner KL, Terman GW, Voepel-Lewis T, Domino KB. Postoperative opioidinduced respiratory depression: a closed claims analysis. Anesthesiology 2015;122:659-65.
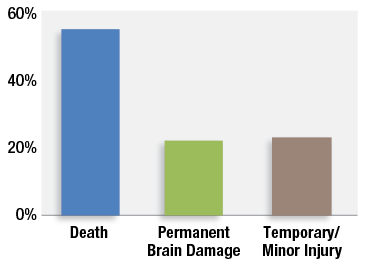
Figure 1: Severity of injury in 92 claims associated with postoperative opioid-induced ventilatory impairment from the Closed Claims Project.
Postoperative opioid-induced ventilatory impairment (OIVI) is a preventable cause of high severity injuries to patients and many organizations have focused efforts on this patient safety issue over the last two decades. Progress has been slow in this arena because the low incidence of these events has made outcomes research on specific interventions difficult. The Anesthesia Closed Claims Project utilizes one method to study these rare events by rigorous examination of factors associated with closed anesthesia malpractice claims from professional liability companies that cover approximately one third of anesthesiologists in the United States. The Closed Claims Project identified 92 claims associated with OIVI.1 Its methodology did not identify the cases where there was no harm from a respiratory event and no claim was filed (e.g., a successful, quick rescue with naloxone), a misdiagnosis as to cause of death or brain injury, the large number of cases that were never pursued in a medicolegal setting,2 or the cases covered by professional liability companies outside of the Closed Claims Project. Over three-quarters of these 92 OIVI claims involved death or permanent brain damage (Figure 1).1
Because of the high severity of injuries related to this complication, many institutional, professional society, and standards-setting organizations have produced guidelines that recommend enhanced postoperative monitoring for high-risk patients receiving postoperative opioids. These guidelines include interventions such as increased assessment checks over shorter intervals, continuous capnography and/or continuous pulse oximetry with centralized alarms, and newer technologies such as the use of electrical impedance to monitor minute ventilation.3,4 These recommendations are a logical start to this complicated problem; however, identifying all patients at high risk for OIVI is not a simple task. Published studies on this topic using different methodologies and databases have identified numerous risk factors for postoperative OIVI including older age, female sex, obesity, underweight, obstructive sleep apnea, renal impairment, cardiac disease, chronic obstructive pulmonary disease, neurologic disease, diabetes, hypertension, chronic use of opioids preoperatively, and airway surgery.5-9 Two-thirds of the 92 claims associated with postoperative opioid-induced respiratory depression in the Closed Claims Project were associated with obesity, though 63% were classified as relatively healthy with ASA Physical Status 1-2.1Specific gene polymorphisms that alter the metabolism and transport of opioids are increasingly being identified and associated with OIVI.7,10,11 Clearly, many of these risk factors will be undiagnosed, reducing the accuracy of any potential risk factor checklist. Moreover, postoperative complications that may evolve such as sepsis, acute kidney injury, pneumonia, delirium, and others may influence a patient’s susceptibility to OIVI.
Exogenous risk factors for this complication are dependent on the practices and policies of health care professionals and institutions and are equally as important as pre-existing patient conditions. Risk factors that have been cited include the use of general anesthesia compared to neuraxial anesthesia, preoperative administration of long-acting oxycodone or gabapentin, continuous infusion of opioids postoperatively, concomitant administration of other non-opioid sedating medications, multiple postoperative prescribers, and inadequate health care provider education regarding the signs and symptoms of OIVI.1,12-14 These exogenous risk factors are highly dependent on the skills, experience, and education of each health care professional involved in a patient’s care throughout their admission, and the integration and communication between all health care providers, especially when new care guidelines are instituted. Institutional resources such as nurse-to-patient staffing ratios on floors, ongoing provider education at all levels for the signs and symptoms of OIVI, computerized order entry, enhanced electronic monitoring with centralized alarms, and institutional policies surrounding pain management are other significant variables that may influence the incidence of this complication.
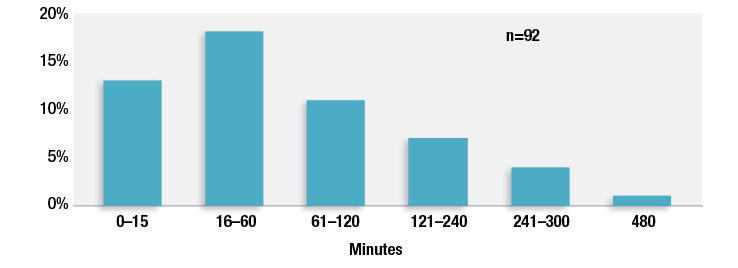
Given this extensive list of known and unknown contributory factors for postoperative OIVI, health care providers and institutions cannot possibly accurately identify all patients who will develop this complication. As the population ages and the obesity and opioid epidemics continue to escalate, and hospital providers care for patients with higher acuity illnesses than in the past, it is likely that the majority of patients will have one or more of these risk factors for OIVI. The recommendation from the APSF and other organizations to institute continuous electronic monitoring for all patients receiving opioids postoperatively would mitigate harm attributable to undiagnosed patient risk factors and variable provider and institutional risk factors.15 It would avoid confusion surrounding identification of high-risk patients and promote standardization of postoperative care for all patients. As nurses care for more patients, using continuous electronic monitoring of patients with centralized alarms will provide more objective and continuous monitoring of patients. Our study demonstrated that almost one third of the 92 claims associated with postoperative OIVI were discovered to have their critical OIVI event within one hour of their last nursing check and 42% within two hours of their last nursing check (Figure 2).1 Fluctuating patient conditions and inadequate education for nurses regarding signs and symptoms of OIVI contributed to these findings. These short time intervals argue that physical nursing assessments alone on the floor are not sufficient to detect OIVI when nurses are caring for more than one patient at a time.
Reproduced and modified with permission. Lee LA, Caplan RA, Stephens LS, Posner KL, Terman GW, Voepel-Lewis T, Domino KB. Postoperative opioidinduced respiratory depression: a closed claims analysis. Anesthesiology 2015;122:659-65.
Figure 2: Time between last nursing check and discovery of opioid-induced ventilatory impairment in 92 claims. Claims with unknown timing (n = 39) and not applicable (at home, n = 3) not shown.
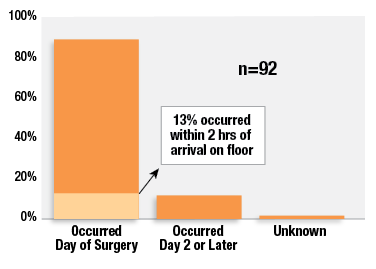
The critical time period for use of continuous electronic postoperative monitoring is primarily within the first 24 hours postoperatively as data from the Closed Claims Project demonstrate that 88% of these events occurred within that time frame (Figure 3).1 Moving from the noisier and higher stimulation area of the recovery room with 1:1 or 1:2 nurse-to-patient ratios to the floor where patients will have less stimulation and less intensive monitoring by nurses is a high-risk time. Our study revealed that 13% of these OIVI events occurred within two hours of moving to the floor. These findings are consistent with other studies that have found that the first 24 hours is the highest risk period for OIVI for postoperative patients.16-18
Reproduced and modified with permission. Lee LA, Caplan RA, Stephens LS, Posner KL, Terman GW, Voepel-Lewis T, Domino KB. Postoperative opioidinduced respiratory depression: a closed claims analysis. Anesthesiology 2015;122:659-65.
Figure 3: Postoperative timing of opioid-induced respiratory depression in 92 claims from the Closed Claims Project.
Lastly, continuous electronic monitoring with centralized alarms would theoretically be able to alert providers of other evolving postoperative complications that can alter respiratory and heart rates and oxygen saturation such as sepsis, hypovolemic shock, pneumonia, and other illnesses. Taenzer and colleagues successfully demonstrated this concept when they instituted electronic surveillance with continuous pulse oximetry with centralized alarms.19,20They noted a significant reduction in ICU transfers from the floor by 50%, a reduction in rescue events by 60% from baseline, and decreased mortality from opioid-related causes. The economic return on investment was also highly significant with an estimated savings of $1.48 million from reduced ICU transfers within their initial study unit.21 This figure did not take into account any potential reduction in lifelong expenses for patients from reduced morbidity or for institutional medicolegal defense. Data from the first 24 hours and further could be utilized to determine when a patient can be weaned from continuous electronic monitoring.
In summary, risk stratification for OIVI is important for perioperative management of anesthetics and medications, but it cannot be done with high reliability. The concept of using only pre-existing patient conditions and illnesses for identifying which patients require continuous electronic monitoring postoperatively negates the significant impact that the health care setting (providers and institution) places on patients for development of OIVI in a variable fashion. Continuous electronic monitoring of oxygenation and/or ventilation for all postoperative patients receiving opioids for at least the first 24 hours would simplify and standardize postoperative care and potentially reduce the incidence of postoperative OIVI and other complications. Initial efforts in resource-limited institutions to increase monitoring for patients for OIVI may focus on patient risk factors, but organizations should aim for the ultimate goal of monitoring all patients receiving opioids postoperatively.
Dr. Lee is a member of the editorial board for the APSF Newsletter and is a staff anesthesiologist with Premier Anesthesia at Kadlec Regional Medical Center in Richland, WA.
Dr. Posner is currently Research Professor and Laura Cheney Professor of Anesthesia Patient Safety in the Department of Anesthesiology and Pain Medicine at the University of Washington in Seattle, WA.
Dr. Domino is Professor of Anesthesiology at the University of Washington in Seattle, WA.
References
- Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology 2015;122:659–65.
- Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med 1991;325:245–51.
- Joint Commission Enhances Pain Assessment and Management Requirements for Accredited Hospitals. The Joint Commission Perspectives 2017;37:1-4. Available at https://www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF Accessed Dec 3, 2017.
- Center for Clinical Standards and Quality/Survey & Certification Group. Memorandum for requirements for hospital medication administration, particularly intravenous (IV) medications and post-operative care of patients receiving IV opioids. Center for Medicare and Medicaid Services. March 14, 2014. https://www.cms.gov/Medicare/Provider-
Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-
Letter-14-15.pdf. Accessed Dec 3, 2017. - Gupta K, Prasad A, Nagappa M, et al. Risk factors for opioid-induced respiratory depression and failure to rescue: a review. Curr Opin Anaesthesiol 2018;31:110-119.
- Khelemsky Y, Kothari R, Campbell N, et al. Incidence and demographics of post-operative naloxone administration: a 13-year experience at a major tertiary teaching institution. Pain Physician 2015;18:E827–9.
- Niesters M, Overdyk F, Smith T, et al. Opioid-induced respiratory depression in paediatrics: a review of case reports. Br J Anaesth 2013;110:175–82.
- Chidambaran V, Olbrecht V, Hossain M, et al. Risk predictors of opioid-induced critical respiratory events in children: naloxone use as a quality measure of opioid safety. Pain Med 2014;15:2139–49.
- Pawasauskas J, Stevens B, Youssef R, et al. Predictors of naloxone use for respiratory depression and oversedation in hospitalized adults. Am J Health Syst Pharm 2014;71:746–50.
- Chidambaran V, Venkatasubramanian R, Zhang X, et al. ABCC3 genetic variants are associated with postoperative morphine-induced respiratory depression and morphine pharmacokinetics in children. Pharmacogenomics J 2017;17:162–169.
- Sadhasivam S, Chidambaran V, Zhang X, et al. Opioid-induced respiratory depression: ABCB1 transporter pharmacogenetics. Pharmacogenomics J2015;15:119–26.
- Weingarten TN, Jacob AK, Njathi CW, et al. Multimodal analgesic protocol and postanesthesia respiratory depression during phase 1 recovery after total joint arthroplasty. Reg Anesth Pain Med 2015;40:330–6.
- Cavalcante AN, Sprung J, Schroeder DR, et al. Multimodal analgesic therapy with gabapentin and its association with postoperative respiratory depression. Anesth Analg 2017;125:141–146.
- George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag 2010;6:47–54.
- Stoelting RK and Overdyk FJ for the Anesthesia Patient Safety Foundation. Conclusions and Recommendations from the June 8, 2011, Conference on Electronic Monitoring Strategies (Essential Electronic Monitoring Strategies to Detect Clinically Significant Drug-Induced Respiratory Depression in the Postoperative period). Available at https://www.apsf.org/initiatives.php?id=10 (last accessed Dec 3, 2017).
- Taylor S, Kirton OC, Staff I, et al. Postoperative day one: a high risk period for respiratory events. Am J Surg 2005; 190:752–6.
- Ramachandran SK, Haider N, Saran KA, et al. Life-threatening critical respiratory events: a retrospective study of postoperative patients found unresponsive during analgesic therapy. J Clin Anesth 2011;23:207–13.
- Weingarten TN, Herasevich V, McGlinch MC, et al. Predictors of delayed postoperative respiratory depression assessed from naloxone administration. Anesth Analg 2015;121:422–9.
- Taenzer AH, Pyke JB, McGrath SP, et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology 2010;112:282–7.
- McGrath SP, Taenzer AH, Karon N, et al. Surveillance Monitoring management for general care units: strategy, design, and implementation. Jt Comm J Qual Patient Saf 2016;42:293–302.
- Taenzer AH, Blike GT. Postoperative monitoring—the Dartmouth experience. APSF Newsletter 2012;27:1. Available at https://www.apsf.org/newsletters/html/2012/spring/01_postop.htm. Accessed Dec 4, 2017.


 Issue PDF
Issue PDF