
Figure 1: Normal EZ-100 Power Sprayer.
Topical anesthesia of the upper airway for awake intubation is often accomplished by spraying local anesthetics through an atomizer.1 In our institute, we routinely utilize EZ-SprayTM (Figure 1) (Alcove Medical Inc., Saratoga Springs, UT). Here we report an event in which the nozzle part of the EZ-SprayTM unexpectedly broke off during routine topicalization for an awake intubation. In this situation, the EZ-SprayTM was powered by 15 L/min of oxygen delivered from an E O2 cylinder. Immediately after the breakage, patient spit out two components of the EZ-SprayTM(Figure 2). Since these two pieces were the only pieces missing and were retrieved in full, and the patient was not coughing and reported no feelings of foreign body in his throat, no imaging of the chest or bronchoscopic exam of his tracheobronchial tree were performed. The originally planned awake intubation and the intended procedure were subsequently accomplished uneventfully. Fortunately, this event resulted in no harm to the patient; however, we would like to take this opportunity to raise the awareness of this potential equipment malfunction. To avoid any related aspiration, we recommend:
| 1. | Check the integrity of the atomizer before spraying. | |
| 2. | Search for the small components shown in the picture if breakage does happen. | |
| 3. | If a part is missing: | |
| A) | The patient’s oral cavity should be thoroughly searched for any residual components of breakage. | |
| B) | If all components cannot be located, a radiograph of the oropharynx, and/or lung should be obtained, and a bronchoscopic examination should be performed which should reveal the metal and plastic components if they have been aspirated, and allow for extraction. | |
| 4. | We also recommend reviewing similar equipment failure related events and reporting them in FDA’s Medsun system. (https://www.fda.gov/MedicalDevices/Safety/MedSunMedicalProductSafety Network/default.htm) |
|
Dr. Mi Wang is a staff anesthesiologist in the Department of Anesthesiology at the Cleveland Clinic, Cleveland, OH.
Dr. Piyush Mathur is a staff anesthesiologist in the Department of Anesthesiology at the Cleveland Clinic, Cleveland, OH.
Dr. Basem Abdelmalak is Professor of Anesthesiology and Director of Anesthesia for Bronchoscopic Surgery and the Center for Sedation in the Department of Anesthesiology at the Cleveland Clinic, Cleveland, OH.
None of the authors have any conflicts of interest as they relate to this article.
Reference
- Collins SR, Randal S. Fiberoptic intubation: an overview and update discussion. Respiratory Care 2014;59:865-880.
Reply:
Alcove Medical, Inc., received notification of the occurrence cited by the Cleveland Clinic providers on June 13, 2017. However, our initial response was based on the understanding that the EZ Spray product referred to was an EZ-103-A, a reposable power sprayer. After receiving photographs of the Power Sprayer involved, however, we now realize Dr. Wang was referring to our EZ-100, a one-time use Power Sprayer.
From Dr. Wang’s report, it seems apparent that somehow the EZ-100 Power Sprayer used by Dr. Wang was not bonded properly. If properly bonded, it would be impossible for the plunger rod and spring to be ejected.
We have since reviewed our assembly safety and inspection procedures and have added and implemented the following: Instead of two tests for function and one for visual quality we have instituted a third “hands on test” where all bonded joints are manually checked for adhesion and strength. Then, they are visually inspected before being processed for shipment.
We believe this safety policy will help insure increased quality and safety in all of our products.
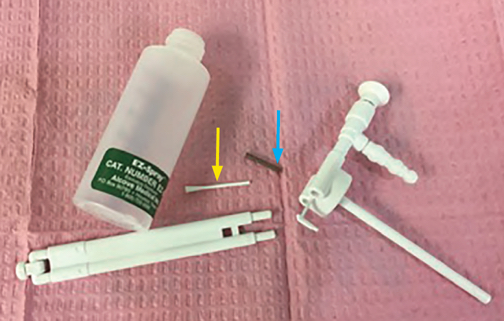
Figure 2: This figure represents the components of the EZ-SprayTM atomizer. The Blue arrow points to the spring, while the yellow arrow points to the pushrod. Both small components were spit out by the patient after EZ-SprayTM breakage.
Our single use power sprayer, EZ-100, was designed specifically for difficult airway management. Its single use, patented, closed system prevents the possibility of cross-contamination and provides the benefit of deep, penetrating atomization. The EZ-100 nozzle extender is bonded to the body. The whole Power Sprayer is discarded after each use.
Alcove Medical is an American-based family business and has never before had any incident of any kind with any of its products since its inception in 1997. We appreciate the opportunity to respond to this matter and to be able to explain the procedures and safety policies put in place in regards to EZ-100 Power Sprayer assembly and all Alcove products.
Thank you,
John K. Bullock, COO
Alcove Medical, Inc.
The information provided is for safety-related educational purposes only, and does not constitute medical or legal advice. Individual or group responses are only commentary, provided for purposes of education or discussion, and are neither statements of advice nor the opinions of APSF. It is not the intention of APSF to provide specific medical or legal advice or to endorse any specific views or recommendations in response to the inquiries posted. In no event shall APSF be responsible or liable, directly or indirectly, for any damage or loss caused or alleged to be caused by or in connection with the reliance on any such information.


 Issue PDF
Issue PDF