Introduction
In U.S. acute care hospitals, 3.2% of patients develop one or more health care-associated infections (HAIs) resulting in increased patient morbidity, mortality, duration of hospitalization, and health care costs.1 Catheter-related bloodstream infections (CRBSIs) are the most common etiology of HAI and these can occur with central as well as peripheral intravascular catheters.1 Each year in the U.S., there are approximately 250,000 CRBSIs from short- and long-term intravascular catheters resulting in significant morbidity, including sepsis, other complications, and mortality.1 In 2014, U.S. acute care hospitals had over 31,000 patients with central-line-associated bloodstream infections (CLABSIs), with an estimated annual cost of $0.6–2.7 billion and a mortality rate of 12–25%.1 CLABSI rates have generally ranged from 1.1–2.5/1000 catheter-days, and each CLABSI on average increases the hospital stay by 10.4 days and adds more than $45,000 in costs.1 In a review of short-term peripheral intravenous catheter (PIVC) CRBSIs from 1980 to 2106, the infection incidence (not reported per 1000 catheter-days) was 1.8 infections/1000 catheters. The arterial catheter CRBSI rate in a 2014 study was 1.26/1000 catheter-days, with the CRBSI risk for the femoral site 1.9 times higher than that of the radial site.1 Microbial contamination of intravascular catheters may occur from either 1) an extraluminal route involving distal migration from the insertion site or 2) intraluminal contamination, which can occur when accessing and using these catheters, with less frequent sources from hematogenous spread or contaminated infusate.
Accessing vascular catheters is routine while providing anesthesia and other patient care, but are health care professionals using optimal methods to reduce the risk of vascular catheter access-related HAIs? If hand hygiene and aseptic technique are not used when accessing vascular catheters, intraluminal contamination of injection ports (e.g., open lumen stopcocks [OLSs] and disinfectable needleless closed connectors [DNCCs]) with microbial pathogens may occur that may lead to CRBSIs and other HAIs.1-3 Unfortunately, low hand hygiene compliance rates have been reported for anesthesia professionals with ranges from 2.9% to 18%.1,4 Syringes and infusions can become contaminated during medication preparation and clinical use with resultant injection of contaminated contents into the bloodstream as well as contamination of access ports.1,2,5-7 Increased use of manufacturer or pharmacy-prepared medication syringes and infusions is recommended by the Anesthesia Patient Safety Foundation, the Association for Professionals in Infection Control and Epidemiology, and the Institute for Safe Medication Practices to decrease contamination and medication errors during preparation and administration of medications and fluids.1,2,5,6 The Centers for Disease Control and Prevention also provides recommendations for Safe Injection Practices.7 This article will compare and contrast contamination and infection risks related to the use of OLSs vs. DNCCs, and discuss recommendations as well as unresolved issues concerning DNCC disinfection.
Contamination and Infection Risks of OLSs and Disinfected DNCCs
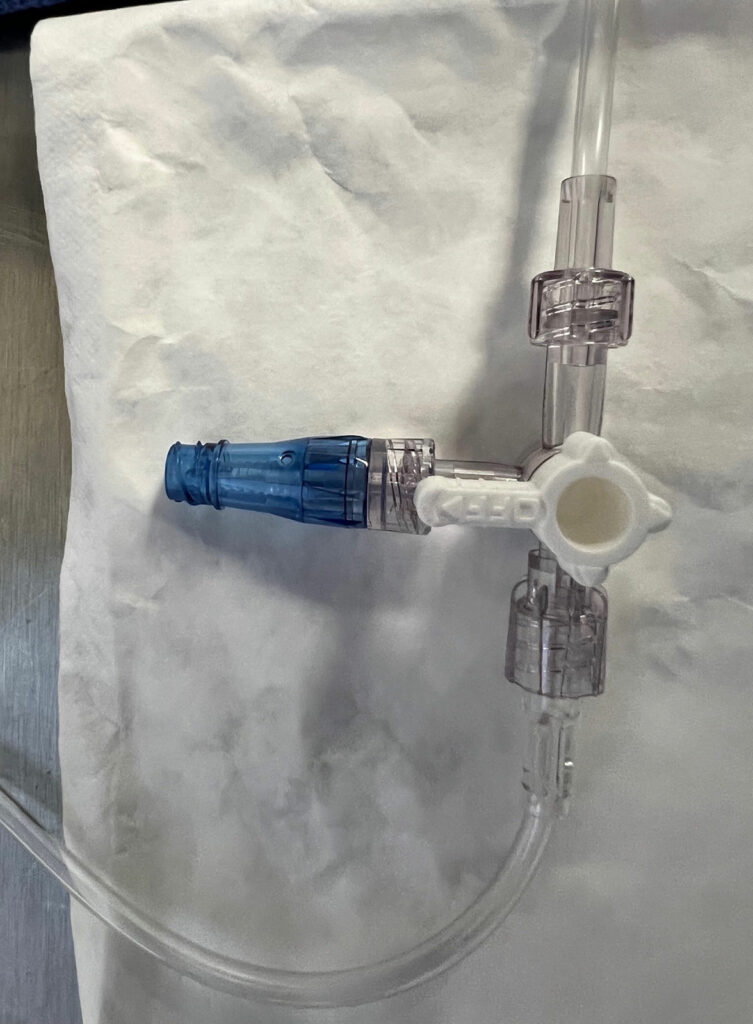
OLSs are commonly used in the practice of anesthesia; however, contamination of intraluminal surfaces may occur in up to 32% to 38% of anesthesia cases.8,9 Neither a 70% isopropyl alcohol (IPA) pad, nor a port-scrub device effectively disinfects an OLS.3,10 An OLS is prone to contamination due to its design with a removable cap which exposes intraluminal surfaces (Figure 1). While over 50% of DNCCs are contaminated with bacteria on their injection surfaces prior to appropriate disinfection,11,12 a DNCC’s injection surface can be disinfected with a high level of effectiveness by scrubbing with an alcohol-containing disinfectant pad or using an IPA cap (Figure 2).1,3,11-17 In a recent critical review of injection ports, 8 of 10 studies had significantly lower rates of intraluminal contamination with disinfected DNCCs compared to OLSs, and 2 of 7 studies had significantly decreased rates of CLABSIs or CRBSIs with disinfected DNCCs compared to OLSs (some studies evaluated both contamination rates and infection rates, while other studies evaluated only a single outcome: either contamination rates or infection rates).1 When examining the subgroup of these studies where both OLSs and DNCCs were disinfected before access, 7 of 9 studies found significantly lower rates of intraluminal contamination with DNCCs compared to OLSs, and 1 of 4 studies had a significantly lower rate of CLABSIs with DNCCs.1 No studies found OLSs were beneficial compared to disinfected DNCCs (See Table 1).1
Table 1: Comparison of Disinfectable Needleless Closed Connectors (DNCCs) to Open Lumen Stopcocks (OLSs)
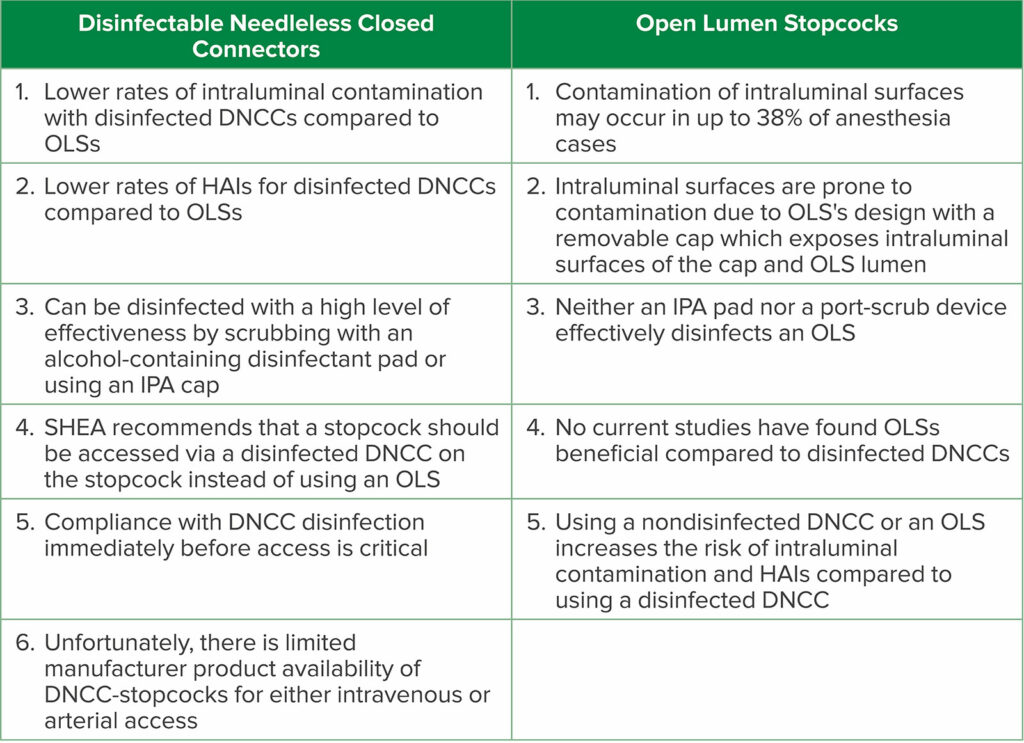
Abbreviations: DNCC-stopcock, stopcock with a DNCC attached (preferably bonded) to the injection lumen; HAIs, health care-associated infections; IPA, 70% isopropyl alcohol; SHEA, Society for Healthcare Epidemiology of America
Microbial Injection and Biofilm
Failure to disinfect DNCCs before access, or contamination of OLSs during clinical use, can lead to intraluminal contamination1,15 resulting in biofilm formation (micro-organisms embedded in an extracellular glycocalyx matrix) on the catheter’s surfaces resulting in an increased risk of HAIs.18,19
Even a single omission of DNCC disinfection before access can result in the formation of biofilm.19 Unfortunately, DNCC disinfection compliance (including hand hygiene and aseptic technique) has been challenging for health care professionals.1,15,20 Although the current literature does not fully explain biofilm’s defense mechanisms against antimicrobial agents, exopolysaccharides impair antibiotics from penetrating the biofilm matrix to reach bacteria in the biofilm.21
Biofilm may also be resistant to the host’s immune system.22,23 Thus, biofilm can be a source of bacteremia and chronic infections.21-23 While in-vitro studies suggest that leukocytes are able to effectively penetrate biofilms, animal studies indicate that biofilm formation results in an “evasion” of the host’s immune response from a “pro-inflammatory, bactericidal response” towards an “anti-inflammatory, pro-fibrotic response.”22 Biofilm formation on surfaces of catheters and other implanted medical devices thus protects the bacteria which encourages the persistence of infection.22
An additional mechanism of microbial injection may explain the association of OLS and DNCC contamination with subsequent increased risk of HAIs.1,14 Inadvertent direct microbial injection into the bloodstream may occur during vascular access via a contaminated OLS or failure to disinfect a DNCC injection surface before injection. Inadvertent direct microbial injection may also occur if a contaminated syringe or infusate is used.1,2,5-7 In an ex-vivo randomized control trial (RCT), conducted during concurrent clinical anesthesia, approximately 10,000 colony forming units of bacteria entered the test circuit per injection when DNCCs or OLSs were not disinfected before access.14 In this study, the incidence of inadvertent bacterial injection was significantly lower when using disinfected DNCC-stopcocks (70% alcohol [method unspecified] with 30 seconds drying) compared to OLSs or DNCC-stopcocks without disinfection.14
Recent Recommendations from the Society for Healthcare Epidemiology of America
The Society for Healthcare Epidemiology of America (SHEA) recently published guidelines for infection prevention in the anesthesia work environment.15 SHEA recommends using disinfected DNCCs on stopcocks for injecting medications as the preferred choice for clinician use rather than using OLSs.24,25 SHEA also noted that “stopcocks on pressure transducers are periodically opened to air to calibrate the transducer” and that “these stopcocks may reasonably be covered with sterile caps rather than needleless injection ports” (DNCCs), but did not comment on a method to maintain intraluminal sterility when the transducer is opened to air.25 It is important that stopcocks used for zeroing pressure transducers maintain intraluminal sterility. While some transducers include a cap with a “small” hole (much smaller than the lumen) which can eliminate the need for cap removal during zeroing, it is unknown whether intraluminal sterility is maintained since this “small” lumen is continuously open to the environment. Furthermore, if this cap is not bonded to the stopcock, the provider might circumvent any potential advantage of this cap’s design by removing the cap during zeroing, thus fully exposing the intraluminal surfaces to potential environmental contamination. A stopcock with a bacterial filter bonded to the zeroing lumen may serve as an alternative option.
Accessing Vascular Catheters via Disinfected DNCCs
The current literature supports that disinfected DNCCs should be used instead of OLSs based on the following premises: the documented overall lower contamination and infection risks of disinfected DNCCs compared to OLSs as discussed above (Table 1) and the recent SHEA recommendations that “stopcocks used for injecting drugs should ideally be closed with needleless injection ports.”25 To reduce infection-related patient risk, vascular catheters used for medication or fluid administration, or blood withdrawal, should be routinely accessed via either disinfected DNCCs (e.g., in intravenous [IV] tubing sets) or via disinfected DNCC-stopcocks. Compliance with disinfection is essential. For DNCC-stopcocks, the DNCC should preferably be bonded to the stopcock injection lumen to eliminate removal and bypassing the DNCC.1 While the recent SHEA recommendations25 do not specifically address using DNCCs to obtain blood samples from arterial pressure tubing, current studies1 support that blood samples from arterial tubing sets be obtained via disinfected DNCC-stopcocks instead of OLSs. The only clinical application where OLSs would not have a greater contamination or infection risk compared to disinfected DNCCs would be when OLS are restricted to use on sterile field related-procedures.1
Limited Manufacturer Availability of DNCC-stopcocks
Presently, IV and arterial transducer tubing sets do not typically come with DNCC-stopcocks.1 Manufacturers should supply IV and arterial transducer tubing sets with DNCC-stopcocks instead of OLSs and there should be DNCC-stopcocks made available as single packaged items. There are several reasons why DNCC-stopcocks are not routinely included in tubing sets including lack of clinician and manufacturer awareness of DNCC superiority, inertia in changing existing practice patterns, and increased cost. Nevertheless, the need to improve safety through adoption of DNCCs is inevitable and the increased cost should not be a barrier. For example, sharps injury prevention safety devices cost more than nonsafety devices, but they are now utilized as standard safety requirements.1,26
Disinfection Method and Type of Disinfectant
The type of disinfectant and disinfection method used on DNCCs are critical factors to maximize the disinfectant’s effectiveness and reduce unwanted HAIs.1,27 The variations in results of several selected in-vitro and clinical studies highlight the difficulties in determining definitive disinfection recommendations (Table 2). Unsurprisingly, a consensus is lacking among experts concerning the recommended disinfectant, the disinfection method (e.g., scrub vs. “clean”), disinfection duration and drying time, and whether to use IPA caps (see Table 3).1 SHEA recommends DNCCs should be disinfected immediately before each access or before a rapid series of injections, such as during anesthesia induction, either by scrubbing (a duration was not specified) with an alcohol-containing disinfectant pad (e.g., IPA or chlorhexidine gluconate [CHG]/IPA), or appropriately utilizing an IPA cap.1,15 Numerous guidelines1 recommend scrubbing with an alcohol-containing disinfectant; however, the recommended scrubbing durations vary from ≥ 5 to ≥ 15 seconds (Table 3).31-33 Since compliance with longer scrub durations is low,15 additional research should identify the minimally effective scrub duration.1 In addition, randomized trials are needed to compare various methods and disinfectants used, since suboptimal DNCC disinfection techniques may increase the risk of HAIs. Further complicating the issue is that the infection risk of disinfected DNCCs may also be related to a variety of injection surface topographies and other design features found in various DNCCs, which can influence disinfection efficacy.12,13,27,35
Table 2: a,bSelected 1) In-Vitro Studies of Disinfection of Contaminated DNCC Surfaces, and 2) Clinical Studies of DNCC Disinfection
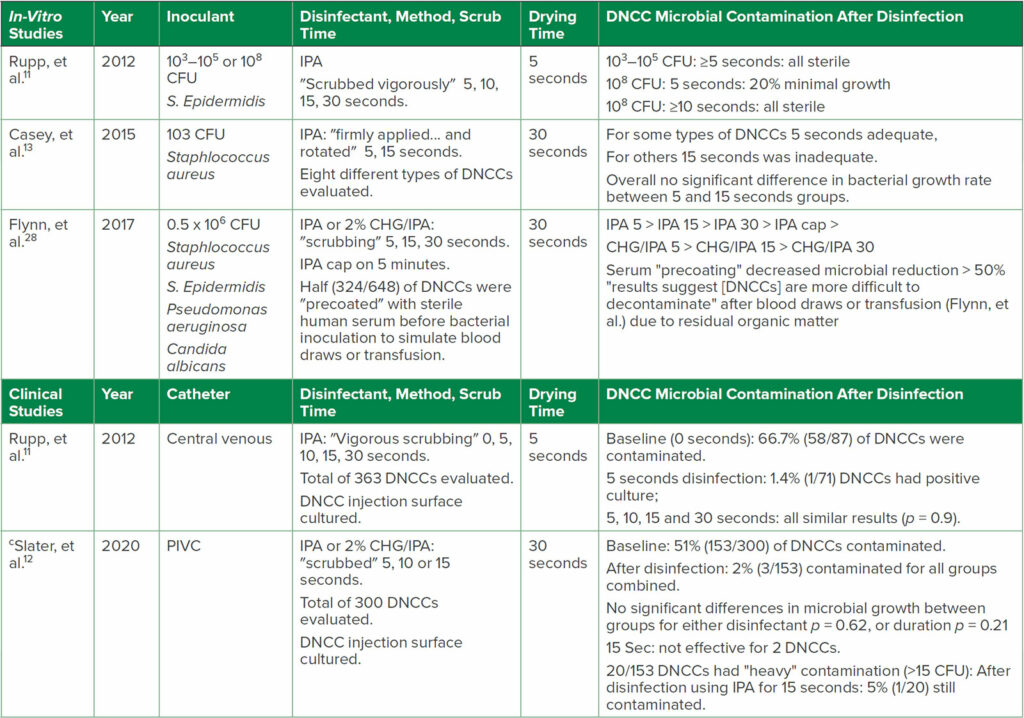
Abbreviations: CFU, colony forming units bacteria/ml inoculant; CHG, chlorhexidine gluconate; DNCCs, disinfectable needleless closed connectors; IPA, 70% isopropyl alcohol; PIVC, peripheral intravenous catheter.
a for additional studies see reference Greene1
b items in quotations are the terminology used in each reference
c first clinical RCT of PIVC DNCC disinfection
Table 3: a,bRecommendations for DNCC Disinfection from Selected National and International Organizations
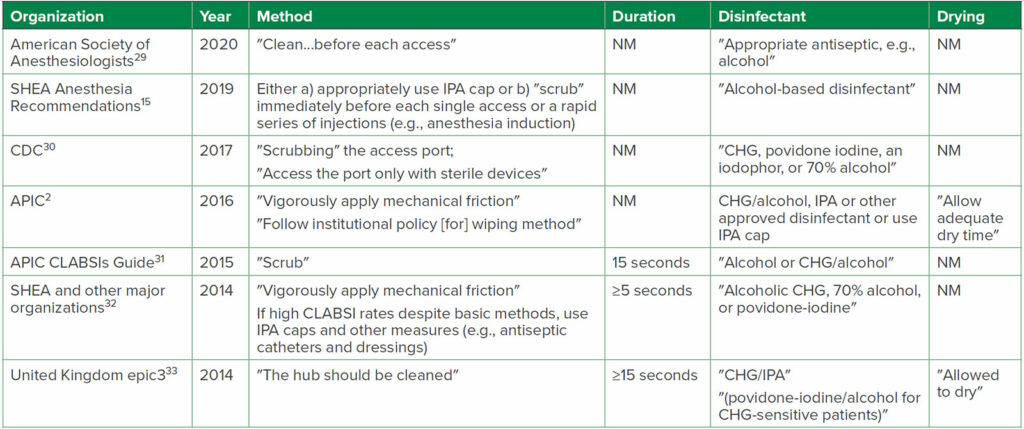
Abbreviations: APIC, Association for Professionals in Infection Control and Epidemiology; CDC, U.S. Centers for Disease Control and Prevention; CHG, chlorhexidine gluconate; CLABSI, central-line-associated bloodstream infection; IPA, 70% isopropyl alcohol; NM, not mentioned in Recommendation; SHEA, Society for Healthcare Epidemiology of America
a for additional Guidelines see references Greene,1 Hallam34
b items in quotations are the terminology used in each reference; i.e., not all stated ″scrub″ as the Method used
Disinfectant Drying Time
A recent study suggested that the disinfectant used on DNCCs should dry before access to reduce the microbial load and its potential for entering the bloodstream.36 Disinfectant drying times vary after scrubbing DNCCs: IPA dries in 5 seconds and CHG/IPA dries in 20 seconds, but povidone-iodine is not dry after 6 minutes.36 However, only a few national and international guidelines mention a need for disinfectant drying (Table 3).1 Unfortunately, only some clinical and in-vitro studies state the drying time after DNCC disinfection, and none compared the effect of various drying times, or no drying at all, on disinfection efficacy.1 Of the 21 studies evaluating DNCC disinfection assessed in a recent critical review, one study specified a 5-second drying time, 10 studies used ≥ 30 seconds drying, and 10 studies did not specify whether drying was used or not.1 Further trials need to address the optimal drying time in the perioperative setting so that health care professionals have clarity on how to reduce infection risk.
Is Injecting Disinfectant into a DNCC Hazardous?
A recent study recommended the DNCC should dry before access to avoid injecting disinfectant.36 An unanswered question is whether injecting some disinfectant into a DNCC is hazardous. This is of great concern since IPA is mainly metabolized to acetone, which is toxic.16,37,38 Two in-vitro studies compared DNCCs scrubbed with either IPA followed by 15 seconds of drying37 or with CHG/ethanol with 30 seconds of drying time,38 followed by saline injections. These studies suggested that alcohol levels in the test circuit fluids were either undetectable37 or “low”38 (maximum µg per in-vitro injection was < 8% of what would produce the estimated toxic blood concentration threshold in neonates, defined as greater than 0.25 mg/ml). Studies on potential IV injection of alcohol or CHG via DNCCs before disinfectant drying are limited in the current literature and therefore, further studies are needed.
IPA Caps
Several national and international recommendations include the option of using IPA caps on DNCCs since they provide passive disinfection, eliminate manual scrubbing (after a minimum contact time), provide a visible indicator of disinfection, provide a contamination barrier, and may increase disinfection compliance compared to manual disinfection.1,15,17,39 IPA cap use requires at least a minimum contact duration on the DNCC before access (manual scrubbing is needed for shorter durations), allowing the disinfectant to dry before access, and discarding the cap after each single use.
A recent “pilot” RCT found no significant differences in CLABSI rates in adults comparing scrubbing with either IPA or CHG/IPA, or using IPA caps.27 Although the 2019 SHEA recommendations15 referred to using IPA caps as a “best practice,” this 2021 “pilot” study suggests that larger more definitive studies should be performed.27 Two in-vitro studies cautioned against using IPA caps in neonates because injection of saline after IPA cap removal resulted in “significant” levels of IPA in the test circuit fluids.16,37,38 One study found that significant IPA levels in the test circuit fluids occurred after 24 hours of IPA cap use, with even higher IPA levels when the DNCCs were exposed to the IPA caps for 7 days.38 The finding that IPA was injected into the test circuit was also problematic since in one study after IPA cap removal, the DNCC was allowed to dry for 30 seconds before injection.38 Disinfectant caps containing ethanol instead of IPA have been suggested as an alternative to decrease the risk of toxicity in neonates.38
Conclusion
 There are numerous issues to be considered for reducing infection risks when accessing vascular catheters. An OLS’s design results in a high rate of intraluminal microbial contamination during clinical use, and neither an IPA pad nor a port-scrub device effectively disinfects an OLS. In contrast, a DNCC’s injection surface can be disinfected with a high level of effectiveness. Although questions remain as to the optimal disinfectant and method of disinfection, and the optimal DNCC design, multiple studies have found lower rates of intraluminal contamination with disinfected DNCCs compared to OLSs, and some studies have found lower rates of HAIs for disinfected DNCCs compared to OLSs. No current studies have found that OLSs are beneficial compared to disinfected DNCCs. Manufacturers should supply IV tubing sets with DNCCs and DNCC-stopcocks instead of OLSs, and DNCC-stopcocks should also be available as single items. Arterial tubing sets should include a DNCC-stopcock for blood sampling and a device for zeroing the transducer that maintains intraluminal sterility. OLSs should be restricted to use on sterile fields. Health care provider compliance with DNCC disinfection is critical to safe use of DNCCs and should include periodic assessments and re-education on hand hygiene and aseptic technique. Increased use of manufacturer or pharmacy-prepared medications and infusions and use of safe injection practices are also recommended to reduce the risk of vascular access-related HAIs and medication errors. Although a consensus on the optimal approach to DNCC disinfection is lacking and many questions remain, a synthesis of the current literature indicates that immediately before access (or a rapid series of injections) the DNCC should be scrubbed with an alcohol-containing disinfectant for at least 5 seconds (some recommendations are to use ≥15 seconds), or properly use an IPA cap, followed by drying before injection. IPA caps have potential advantages compared to manual scrubbing; however, additional studies are needed to determine whether IPA caps are more effective for reducing HAIs than the present alternative methods, and whether they are safe for use in neonates.
There are numerous issues to be considered for reducing infection risks when accessing vascular catheters. An OLS’s design results in a high rate of intraluminal microbial contamination during clinical use, and neither an IPA pad nor a port-scrub device effectively disinfects an OLS. In contrast, a DNCC’s injection surface can be disinfected with a high level of effectiveness. Although questions remain as to the optimal disinfectant and method of disinfection, and the optimal DNCC design, multiple studies have found lower rates of intraluminal contamination with disinfected DNCCs compared to OLSs, and some studies have found lower rates of HAIs for disinfected DNCCs compared to OLSs. No current studies have found that OLSs are beneficial compared to disinfected DNCCs. Manufacturers should supply IV tubing sets with DNCCs and DNCC-stopcocks instead of OLSs, and DNCC-stopcocks should also be available as single items. Arterial tubing sets should include a DNCC-stopcock for blood sampling and a device for zeroing the transducer that maintains intraluminal sterility. OLSs should be restricted to use on sterile fields. Health care provider compliance with DNCC disinfection is critical to safe use of DNCCs and should include periodic assessments and re-education on hand hygiene and aseptic technique. Increased use of manufacturer or pharmacy-prepared medications and infusions and use of safe injection practices are also recommended to reduce the risk of vascular access-related HAIs and medication errors. Although a consensus on the optimal approach to DNCC disinfection is lacking and many questions remain, a synthesis of the current literature indicates that immediately before access (or a rapid series of injections) the DNCC should be scrubbed with an alcohol-containing disinfectant for at least 5 seconds (some recommendations are to use ≥15 seconds), or properly use an IPA cap, followed by drying before injection. IPA caps have potential advantages compared to manual scrubbing; however, additional studies are needed to determine whether IPA caps are more effective for reducing HAIs than the present alternative methods, and whether they are safe for use in neonates.
Elliott S. Greene, MD, is a professor of anesthesiology in the Department of Anesthesiology at Albany Medical College, Albany, NY.
The author has no conflicts of interest.
References
- Greene ES. Challenges in reducing the risk of infection when accessing vascular catheters. J Hosp Infect. 2021;113:130–144.
- Dolan SA, Arias KM, Felizardo G, et al. APIC position paper: Safe injection, infusion, and medication vial practices in health care. Am J Infect Control. 2016;44:750-757.
- Holroyd JL, Paulus DA, et al. Universal intravenous access cleaning device fails to sterilize stopcocks. Anesth Analg. 2014;118:333-343.
- Rowlands J, Yeager MP, Beach M, et al. Video observation to map hand contact and bacterial transmission in operating rooms. Am J Infect Control. 2014;42:698–701.
- Institute for Safe Medication Practices Safe Practice Guidelines for Adult IV Push Medications. https://www.ismp.org/sites/default/files/attachments/2017-11/ISMP97-Guidelines-071415-3.%20FINAL.pdf Last accessed October 10, 2021.
- Anesthesia Patient Safety Foundation. Recommendations for improving medication safety consensus from four work groups at the 2018 APSF Stoelting Conference on Medication Safety. Rochester, MN: APSF; 2018. https://www.apsf.org/medication-safety-recommendations Last accessed October 10, 2021.
- Centers for Disease Control and Prevention. Safe injection practices to prevent transmission of infections to patients. Atlanta, GA. https://www.cdc.gov/injectionsafety/ip07_standardprecaution.html Last accessed October 1, 2021.
- Mermel LA, Bert A, Chapin KC, LeBlanc L. Intraoperative stopcock and manifold colonization of newly inserted peripheral intravenous catheters. Infect Control Hosp Epidemiol. 2014;35:1187–1189.
- Loftus RW, Koff MD, Burchman CC, et al. Transmission of pathogenic bacterial organisms in the anesthesia work area. Anesthesiology. 2008;109:399–407.
- Loftus RW, Brindeiro BS, Kispert DP, et al. Reduction in intraoperative bacterial contamination of peripheral intravenous tubing through the use of a passive catheter care system. Anesth Analg. 2012;115:1315–1323.
- Rupp ME, Yu S, Huerta T, et al. Adequate disinfection of a split-septum needleless intravascular connector with a 5-second alcohol scrub. Infect Control Hosp Epidemiol. 2012;33:661–665.
- Slater K, Cooke M, Fullerton F, et al. Peripheral intravenous catheter needleless connector decontamination study – randomized controlled trial. Am J Infect Control. 2020;48:1013–1018.
- Casey A, Karpanen T, Nightingale P, Elliott T. An in vitro comparison of microbial ingress into 8 different needleless IV access devices. J Infus Nurs. 2015;38:18–25.
- Loftus RW, Patel HM, Huysman BC, et al. Prevention of intravenous bacterial injection from health care provider hands: the importance of catheter design and handling. Anesth Analg. 2012;115:1109–1119.
- Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area. Infect Control Hosp Epidemiol. 2019;40:1–17.
- Voor In’t Holt AF, Helder OK, Vos MC, et al. Antiseptic barrier cap effective in reducing central line-associated bloodstream infections: a systematic review and meta-analysis. Int J Nurs Stud. 2017;69:34–40.
- Merrill KC, Sumner S, Linford L, et al. Impact of universal disinfectant cap implementation on central line associated bloodstream infections. Am J Infect Control. 2014;42:1274–1277.
- Mermel LA. What is the predominant source of intravascular catheter infections? Clin Infect Dis. 2011;52:211–212.
- Moureau NL, Flynn J. Disinfection of needleless connector hubs: clinical evidence systematic review. Nurs Res Pract. 2015;2015:796762.
- Ryan AJ, Webster CS, Merry AF, Grieve DJ. A national survey of infection control practice by New Zealand anaesthetists. Anaesth Intensive Care. 2006;34:68e74
- Balikci E, Yilmaz B, Tahmasebifar A, et al. Surface modification strategies for hemodialysis catheters to prevent catheter-related infections: a review. J Biomed Mater Res B Appl Biomater. 2021;109:314-327.
- Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16:397–409.
- Gominet M, Compain F, Beloin C, Lebeaux D. Central venous catheters and biofilms: where do we stand in 2017? Acta Pathol Microbiol Immunol Scand. 2017;125:365–375.
- Greene ES. New SHEA expert guidance for infection prevention in the anesthesia work area needs improvement. Infect Control Hosp Epidemiol. 2019;40:607–608.
- Deloney V, Bowdle A, Birnbach DJ, et al. Reply to Greene: New SHEA expert guidance for infection prevention in the anesthesia work area needs improvement. Infect Control Hosp Epidemiol. 2021;25:1.
- US Occupational Safety and Health Administration. Bloodborne pathogens and needlestick prevention. Washington, DC. https://www.osha.gov/SLTC/bloodbornepathogens/index.html. Last accessed October 1, 2021.
- Rickard CM, Flynn J, Larsen E, et al. Needleless connector decontamination for prevention of central venous access device infection: a pilot randomized controlled trial. Am J Infect Control. 2021;49:269–273.
- Flynn JM, Rickard CM, Keogh S, Zhang L. Alcohol caps or alcohol swabs with and without chlorhexidine: an in vitro study of 648 episodes of intravenous device needleless connector decontamination. Infect Control Hosp Epidemiol. 2017;38:617–619.
- Practice guidelines for central venous access 2020: an updated report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2020;132:8–43.
- O’Grady NP, Alexander M, Burns LA, et al. Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. Atlanta, GA: Centers for Disease Control and Prevention; updated October 2017. Available at: https://www.cdc.gov/infectioncontrol/pdf/guidelines/bsi-guidelines-H.pdf Last accessed November 23, 2021.
- Association for Professionals in Infection Control and Epidemiology. Guide to preventing central line-associated bloodstream infections. Washington, DC: APIC; 2015. http://apic.org/Resource_/TinyMceFileManager/2015/APIC_CLABSI_WEB.pdf. Last accessed October 1, 2021.
- Marschall J, Mermel LA, Fakih M, et al. Society for Healthcare Epidemiology of America. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:753–771.
- Loveday HP, Wilson JA, Pratt RJ, et al. UK Department of Health. epic3: National evidence based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2014;86(Suppl. 1):S1–70.
- Hallam C. (2019) Right Hub Disinfection for Compliance. In: Moureau N. (eds) Vessel Health and Preservation: The Right Approach for Vascular Access. pp 235–241 Springer, Cham. https://doi.org/10.1007/978-3-030-03149-7_18. Online June 11, 2019. Last accessed November 22, 2021.
- Casey AL, Karpanen TJ, Nightingale P, Elliott TS. The risk of microbial contamination associated with six different needle-free connectors. Br J Nurs. 2018;27:S18–S26.
- Slater K, Fullerton F, Cooke M, et al. Needleless connector drying time – how long does it take? Am J Infect Control. 2018;46:1080–1081.
- Sauron C, Jouvet P, Pinard G, et al. Using isopropyl alcohol impregnated disinfection caps in the neonatal intensive care unit can cause isopropyl alcohol toxicity. Acta Paediatr. 2015;104:e489–493.
- Hjalmarsson LB, Hagberg J, Schollin J, Ohlin A. Leakage of isopropanol from port protectors used in neonatal care-Results from an in vitro study. PLoS One. 2020;15:e0235593.
- Bell T, O’Grady NP. Prevention of central line-associated bloodstream infections. Infect Dis Clin N Am. 2017;31:551–559.


 Issue PDF
Issue PDF PDF
PDF