We report a case of a 55-year old male who developed cardiac arrest precipitated by the development of an acute intraoral kink of a polyvinyl endotracheal tube. The physiologic mechanism is postulated to be rapid, and sustained, increased intrathoracic pressures with resultant escalation in vagal activity exacerbating preexisting bifascular block with progression to complete atrioventricular block. Although rare, polyvinyl endotracheal tube occlusion caused by kinking may cause profound physiologic perturbations and as such requires prompt identification followed by the rapid restoration of a patent airway.
Dear Rapid Response:
A 55-year-old male with a history of pre-existing bifasicular heart block was orally intubated uneventfully with a 7.5 millimeter Shiley polyvinyl endotracheal tube (ETT) (Covidien LLC, Mansfield, MA) for elective endoscopic sinus surgery. The ETT was secured in a neutral position with a cloth tube tie and supported with an endotracheal tube holder. Anesthetic maintenance was accomplished with a combination of inhaled sevoflurane 0.9 percent augmented with a continuous propofol infusion at 100 microgram per kilogram per minute and remifentanil 0.15 microgram per kilogram per minute. The patient was ventilated using an Aisys CS² Anesthesia Delivery System (GE Healthcare, Chicago, IL) with volume control mode settings of 450 milliliter of tidal volume, positive end expiratory pressure of 5 centimeters of water (cm H2O), respiratory rate of 12, and an inspiratory to expiratory ratio of 1:2 with pressure limit set to 40 cm H2O. After 120 minutes following surgical start, the patient developed an acute sustained elevation in peak airway pressure (PIP) from 33 cm H20 to 62 cm H20. This pressure rise immediately preceded the development of a third-degree atrioventricular (AV) block, which progressed to cardiac arrest. Cardiopulmonary resuscitation was performed, and the patient ultimately stabilized with an epinephrine infusion. Post arrest respiratory compliance remained poor necessitating an increase in the pressure limit to allow for PIP persistently greater than 40 cm H20, despite reduction in tidal volume to 4 milliliter per kilogram, muscle relaxation, and prolongation of inspiratory time. A bronchoscope was unable to be advanced through the ETT due to near complete luminal occlusion secondary to ETT distortion (Panel A) with subsequent video assisted laryngoscopy confirming a kink in the ETT at the 19-centimeter marking (Panel B). After failure to advance a 14 French airway exchange catheter (Cook Medical, Bloomington, IN) due to ETT luminal occlusion, extubation was performed followed by emergent reintubation with immediate normalization of ventilation mechanics.
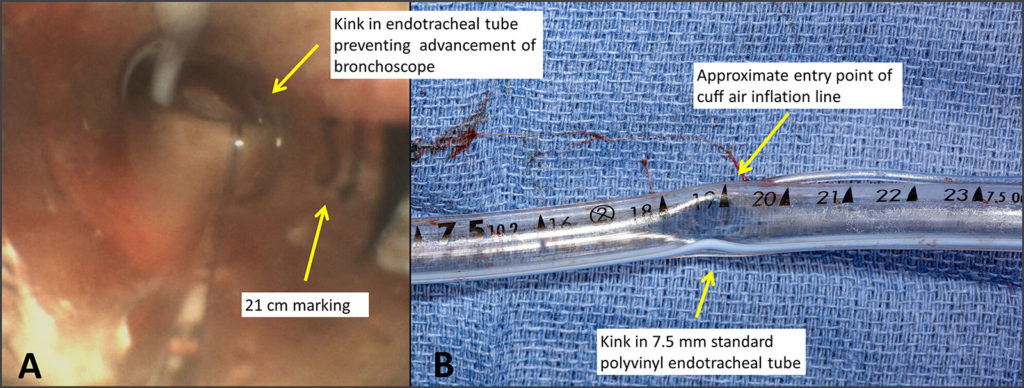
Figure A: Bronchoscopic evaluation demonstrating supraglottic obstruction of the polyvinyl endotracheal tube near the 19-centimeter marking.
Figure B: Photograph of the patient’s 7.5 centimeter polyvinyl endotracheal tube following extubation with an acute angle luminal narrowing evident at the 19-centimeter marking.
Initial post-arrest diagnostic workup was significant for an elevated arterial partial pressure of carbon dioxide of 64 torr, which normalized rapidly after endotracheal tube exchange. Electrocardiogram documented sinus rhythm with bifascicular block while transesophageal echocardiography identified generalized left ventricular hypokinesis with an estimated ejection fraction of 40% and mild-moderately reduced right ventricular systolic function. Chest x-ray was negative for acute findings. Serial cardiac troponins were below the institutional cutoff for myocardial ischemia. Electrolytes were within normal limits with the exception of ionized calcium that was low at 3.75 milligrams per deciliter.
Discussion
An acute increase in intrathoracic pressure can produce increased vagal activity which in turn results in decreased conductance through the AV node.1,2 This process is similar physiologically to the use of a Valsalva maneuver to terminate supraventricular tachycardia.² The postulated mechanism in this patient is the generation of vagally mediated bradycardia precipitated by the acute increase in intrathoracic pressure causing progression of this patient’s bifascicular block to a third-degree AV block. Although it is plausible that air trapping secondary to acute expiratory flow obstruction was causative, the absence of an inspiratory pause to confirm the presence of elevated intrathoracic pressure precludes diagnostic certainty. Consequently, the ultimate etiology of the cardiac arrest remains impossible to ascertain given the multitude of other potentially contributing factors including loss of cardiac output, hypercarbia, hypocalcemia, surgical stress, coronary ischemia, arrhythmia, and concurrent volatile anesthetic agent administration.
ETT kinking is relatively uncommon with the majority of kinks occurring external to the oropharynx such that they are easily identified.³ ETTs are resistant to kinking at room temperature. However, once heated to body temperature, kinking may occur at markedly reduced acuity angles.⁴ The cuff air inflation line has been noted as a point of potential weakness, with others reporting kinking at this location with Mallinckrodt (Tyco Healthcare)⁴ and Rusch (Teleflex)⁵ ETTs. Kinking occurs more frequently with bending in the direction of the convexity of the tube.⁵ The first sign of an endotracheal tube kink may be changes in peak airway pressures or the capnography waveform that may precede the development of hypercarbia and/or hypoxia. Difficulty passing a flexible suction catheter may raise suspicion for an occlusion. In our case, the kink was readily identified via bronchoscopy. After assessing the difficulty of reintubation and obtaining backup airway equipment including supplies for surgical airway, it is recommended to emergently replace the kinked endotracheal tube. Preventative strategies include ensuring the non-traumatic insertion and securing of the ETT while protecting against physical displacement during patient positioning or oropharyngeal surgery. Approaches to mitigate clinically significant physiologic perturbations involve the rapid identification of ETT occlusion to facilitate prompt airway exchange in a controlled setting prior to onset of respiratory and or cardiovascular collapse. Should the surgical scenario require the exit of the ETT at an acute angle, consideration should be given to replacement with a Ring-Adair-Elwyn (RAE) or wire spiral ETT, although the latter carries the potential risk of permanent occlusion should kinking occur due to the reinforced nature of its design. Additionally, should concern for or confirmed kinked ETT occur, established institutional incident reporting mechanisms should be utilized and, if necessary, manufacturer review/correspondence be initiated.
Troy Seelhammer, MD, is an assistant professor of Anesthesiology at the Mayo Clinic, Rochester, MN.
Robert White, MD, is a resident physician in the Department of Anesthesiology at the Mayo Clinic, Rochester, MN.
Roger Hofer, MD, is an assistant professor of Anesthesiology at the Mayo Clinic, Rochester, MN.
The authors have no conflicts of interest.
References
- Alboni P, Holz A, Brignole M. Vagally mediated atrioventricular block: pathophysiology and diagnosis. Heart (British Cardiac Society). 2013;99:904–908.
- Wong LF, Taylor DM, Bailey M. Vagal response varies with Valsalva maneuver technique: a repeated-measures clinical trial in healthy subjects. Ann Emerg Med. 2004;43:477–482..
- Szekely SM, Webb RK, Williamson JA, Russell WJ. The Australian Incident Monitoring Study. Problems related to the endotracheal tube: an analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21:611–616.
- Hübler M, Petrasch F. Intraoperative kinking of polyvinyl endotracheal tubes. Anesth Analg. 2006;103:1601–1602.
- Hariharan U, Garg R, Sood R, Goel S. Intraoperative kinking of the intraoral portion of an endotracheal tube. J Anaesthesiol Clin Pharmacol. 2011;27:290–291.
In Response:
Thank you for reaching out to request the Medtronic response to the report by Drs. Seelhammer, White and Hofer entitled “Cardiopulmonary Arrest Precipitated by Supraglottic Kinking of Polyvinyl Endotracheal Tube”, submitted for publication in the APSF Newsletter.
In assessing the issue raised in the report, senior members of the Medtronic Respiratory Interventions Design, Safety, Post-Market Vigilance (PMV) and Marketing teams, along with the company representative and me, met with the authors. Our aim was to address the concerns raised by the authors, gain deeper understanding of the event, and establish whether this event occurred due to a product design defect. This letter serves as a summary of our discussion with the authors, respectfully submitted to you and the authors in response.
Background Information
The authors describe a case of bronchoscopically confirmed emergent endotracheal tube (ETT) occlusion with subsequent cardiac arrest, during anesthesia. The authors also refer to previous reported cases where kinking occurred at the entry point of the cuff inflation line (which is not where the kinking occurred in the subject case), and raise the question whether efforts to prevent recurrence of this event may require ETT design mitigation. The ETT was not returned to Medtronic for examination, so a picture record of the ETT was supplied to us (and also submitted to you). These photographs show that the kink occurred below (proximal to) and at a point opposite to the inflation line at its entry point into the tube (this is visible in the photo). The authors did not offer an explanation as to how they determined the kinking was due to a design defect, nor did they discuss how they eliminated other possible causes. This event was also submitted via formal complaint to Medtronic, and this discussion will form part of the response to same.
Reported Incidents
Our PMV team have confirmed that between November 2018 and October 2020, Medtronic sold roughly 11.2 million Shiley™ endotracheal tubes. The complaint rate is 0.7 complaints per million ETTs sold during that time period.
Design Discussion
This correspondence arose in response to authors raising the potential of the cuff inflation line being a site of possible kinking. All Shiley™ endotracheal tubes are designed and tested to comply with the requirements of international standard ISO-5361, which provides requirements and guidance to ensure products are designed to be state of the art and meet safety and performance expectations. The standard includes specific requirements regarding tube dimensions and features, as well as specific functional test methods that include a ball/curve test to measure each tube’s resistance to kinking or collapse.
Outcome and Summary
We had a fruitful discussion with Troy Seelhammer, MD, regarding details around this complaint, specifically around the condition of the tube pre-insertion, surgical positioning, and other possible intraoperative events that may have resulted in the kink. Based on this discussion, the pictorial evidence provided, and the documented complaint submitted by the authors, we are confident that the kink did not occur due to a design flaw, and that no design mitigation is necessary.
The Medtronic Mission guides us to strive without reserve for the greatest possible reliability and quality in our products. In order to achieve that outcome, we rely heavily on physicians such as the authors of this paper, and organizations such as the APSF, to ensure that we remain true to this statement. May we therefore take this opportunity to request that, should adverse events occur with any Medtronic product, and where not prohibited by COVID-19 or other guidelines, the actual product (or product of the same lot) be returned to us. This would help immensely in the investigation of reported complaints.
Please feel free to reach out should you have any further questions or concerns.
Respectfully,
Karen A. Phillips, MD, FCA, MBA
Chief Medical Officer, Respiratory Interventions
Consultant Anesthesiologist and Intensivist
Medtronic
Respiratory Interventions Operating Unit
2101 Faraday Ave
Carlsbad, CA 92008
United States
www.medtronic.com
The information provided is for safety-related educational purposes only, and does not constitute medical or legal advice. Individual or group responses are only commentary, provided for purposes of education or discussion, and are neither statements of advice nor the opinions of APSF. It is not the intention of APSF to provide specific medical or legal advice or to endorse any specific views or recommendations in response to the inquiries posted. In no event shall APSF be responsible or liable, directly or indirectly, for any damage or loss caused or alleged to be caused by or in connection with the reliance on any such information.


 Issue PDF
Issue PDF