| The information provided is for safety-related educational purposes only, and does not constitute medical or legal advice. Individual or group responses are only commentary, provided for purposes of education or discussion, and are neither statements of advice nor the opinions of APSF. It is not the intention of APSF to provide specific medical or legal advice or to endorse any specific views or recommendations in response to the inquiries posted. In no event shall APSF be responsible or liable, directly or indirectly, for any damage or loss caused or alleged to be caused by or in connection with the reliance on any such information. |
Supraglottic airway devices (SADs) continue to gain popularity and are increasingly used in anesthetic practices. However, the efficacy and safety of SADs for laparoscopic surgery are disputed. Although not traditionally used in laparoscopic surgery, SADs offer several benefits for appropriately selected patients.
EVOLUTION OF THE SAD
 Since the invention of the first SAD, the device has undergone several design advancements that improve its safety profile.1 The classic laryngeal mask airway developed by Teleflex (Wayne, PA) was one of the first SADs.1 It had a relatively simple design, but it revolutionized the concept of airway management as it allows for a hands-free approach to ventilation and bypasses upper airway obstruction relative to the facemask.1 Innovation has led to the creation of second-generation SADs, which allow for higher oropharyngeal leak pressures.1 This improvement allows for better protection against regurgitated gastric contents and reduces aspiration risk.1-3 In addition, it allows for the delivery of more successful positive pressure ventilation.1,2
Since the invention of the first SAD, the device has undergone several design advancements that improve its safety profile.1 The classic laryngeal mask airway developed by Teleflex (Wayne, PA) was one of the first SADs.1 It had a relatively simple design, but it revolutionized the concept of airway management as it allows for a hands-free approach to ventilation and bypasses upper airway obstruction relative to the facemask.1 Innovation has led to the creation of second-generation SADs, which allow for higher oropharyngeal leak pressures.1 This improvement allows for better protection against regurgitated gastric contents and reduces aspiration risk.1-3 In addition, it allows for the delivery of more successful positive pressure ventilation.1,2
SUPRAGLOTTIC AIRWAY AND HEMODYNAMICS
One potential benefit of SADs in laparoscopic surgery is improved hemodynamic stability.3-5 In a study that assessed hemodynamics and catecholamine levels in obese patients undergoing laparoscopic gastric banding, patients randomized to receive an endotracheal tube (ETT) rather than a SAD had higher blood pressure and higher circulating catecholamine levels throughout the procedure than those in the SAD group.4 High catecholamine levels can increase a patient’s heart rate, which may impair myocardial oxygen delivery.4 They also lead to a prothrombotic state.4 The increase of catecholamines can exacerbate perioperative complications; therefore, SADs are an appealing alternative in certain high-risk populations. Placement of the SAD leads to less sympathetic stimulation and has the potential to require less anesthesia, avoiding reductions in systemic vascular resistance and myocardial depression.5-7 The combination of a catecholamine surge and increased anesthetic requirements for ETTs can further lead to hemodynamic alterations that may not be well tolerated in certain patient populations.
COMPARING SAD VS. ETT OUTCOMES
Another potential benefit of SADs over ETTs is that SADs may be associated with less airway morbidity than the ETT.5,6,8,9 The incidence of sore throat in the ambulatory surgical setting was found to be 45.5% in patients with an ETT compared to 17.5% in patients with an SAD.9 In a meta-analysis of randomized controlled trials comparing the SAD and ETT in patients undergoing elective laparoscopic surgery, there was a higher incidence of laryngospasm, dysphagia, dysphonia, sore throat, and hoarseness in the ETT group.8 Similarly, pediatric patients undergoing anesthesia with recent upper respiratory infections are at an increased risk for respiratory complications, such as bronchospasm and laryngospasm with an ETT vs. a SAD.6,10 When pediatric patients, aged 3 months to 16 years, with a recent upper respiratory infection were randomized to receive a SAD vs. ETT for their anesthetic for a variety of elective surgical procedures, the patients who had an ETT had an increased incidence of bronchospasm and desaturation, defined as SpO2 <90% during airway management as compared to those patients who had a SAD.6 There is a reduced rate of laryngospasm, cough, and desaturation in pediatric patients undergoing laparoscopic hernia repair with SAD placement when compared to ETT placement.11 Data suggests that SAD may reduce the risk of perioperative respiratory complications, even in a high risk group for bronchospasm, laryngospasm, and desaturation.6,11 Furthermore, studies mentioned above suggest reduced patient airway complaints associated with SADs as well as a reduction in airway complications.
The reductions in airway morbidity and fewer hemodynamic disturbances may contribute to earlier discharge times in patients who undergo airway management with SADs.4 In a randomized controlled trial that assessed postanesthesia care unit (PACU) and hospital length of stay, patients who received a SAD during their anesthetic for laparoscopic gastric banding met PACU discharge criteria 17 minutes earlier than those patients who received an ETT for their anesthetic.4
SAD AND VENTILATION DURING PNEUMOPERITONEUM
One of the challenging aspects of laparoscopic surgery is pneumoperitoneum. The physiological changes associated with a pneumoperitoneum may lead to increased abdominal pressure, reduced diaphragmatic excursion, and ultimately reduced respiratory compliance, which hinders the efficacy of ventilation and increases the likelihood of gastric regurgitation and the risk of aspiration.3,12,13 However, newer SADs are designed to allow higher oropharyngeal leak pressure.1,3,8 This is advantageous because it allows for improved ventilation, particularly when implementing positive pressure ventilation.8,14 In a meta-analysis of randomized controlled trials comparing ETT to SAD in patients undergoing laparoscopic surgery, the studies found no difference in the incidence of oropharyngeal leak pressure or desaturation.8 This suggests that effective ventilation is possible with SADs during pneumoperitoneum.3,7,8,14-16 In another meta-analysis comparing randomized controlled trials, case-series, and large prospective observational studies, ventilation was found to be effective in 99.5% of patients with a SAD.14 The only concerning subgroup of patients were those patients with BMI > 30 as they more likely to require ETT placement due to respiratory obstruction or an air leak.14 These studies support the idea that adequate ventilation and oxygenation can be achieved while using a SAD for laparoscopic surgery in nonobese patients.
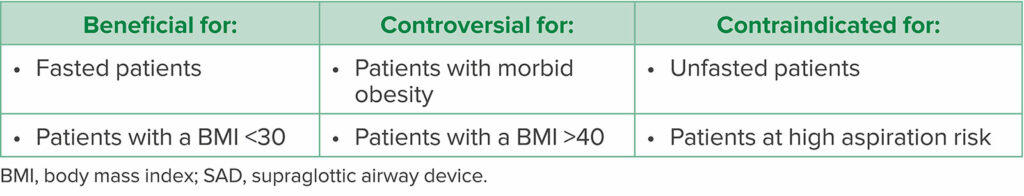
Another commonly cited disadvantage of SADs is gastric insufflation resulting from an insufficient adhesive seal.5 With gastric insufflation there is a risk of aspiration,5 which is one of the most cited contraindications for SAD placement, particularly in patients who are at increased risk (Table 1).17 In patients with a high risk of aspiration, such as unfasted patients and those with a bowel obstruction, it is prudent to continue with ETT intubation. However, there are many studies with successful use of second-generation SADs in laparoscopic surgery without evidence of gastric insufflation or aspiration.7,8,14 One of the greatest determinants of leak and gastric insufflation is the seal and positioning of the SAD.3,5,18 When evaluated after gastric insufflation by a fiberoptic bronchoscope, 44% of first-generation SADs were found to be malpositioned.18 However, properly positioned first-generation SADs showed only a 3% incidence of gastric insufflation.18 Second-generation SADs were designed to reduce the risk of gastric insufflation by allowing for better seals and higher oropharyngeal leak pressures.1,3,18 Thus, second-generation SADs reduce the potential risk of gastric reflux and aspiration when compared to first-generation SADs.2,8,19 In addition, second-generation SADs are equipped with a gastric port that can drain gastric contents from the airway and serve as a conduit for gastric tube placement.1,2 SADs have been successfully used without evidence of aspiration in appropriately selected patients undergoing laparoscopic surgery.15
Table 1: Patient Characteristics Indicating SAD Use14,17,20
CONCLUSION
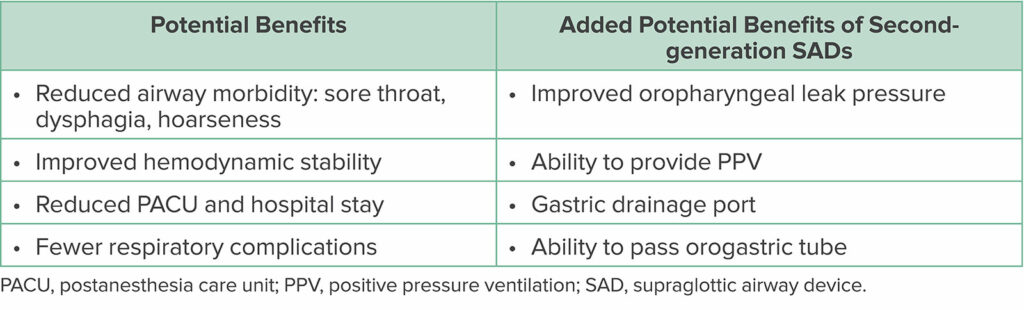
Second-generation SADs are a safe alternative for laparoscopic surgeries in appropriately selected patients. They are better than the first-generation SADs at protecting against gastric insufflation and aspiration. They also have improved ventilation that is effective even with pneumoperitoneum (Table 2). Anesthesia professionals may need to discontinue the use of first-generation devices in laparoscopic surgery due to the lower oropharyngeal leak pressures and increased incidence of gastric insufflation if improperly sealed. Otherwise, SADs may offer a variety of benefits over ETTs in laparoscopic surgery including improved hemodynamic stability, a reduced risk of perioperative respiratory complications, reduced airway morbidity, and they may even contribute to earlier hospital discharge. Second-generation SADs have many benefits that warrant their use in laparoscopic surgery.
Table 2: Potential Benefits of SADs1,2,4,6,9,17
Shauna Schwartz, DO, is a cardiothoracic anesthesiology fellow in the Department of Anesthesiology at the University of Florida College of Medicine.
Yong G. Peng, MD, PhD, FASE, FASA, is a professor of anesthesiology and chief of the Division of Cardiothoracic Anesthesia in the Department of Anesthesiology at the University of Florida College of Medicine in Gainesville, FL.
The authors have no conflicts of interest.
References
- Sharma B, Sahai C, Sood J. Extraglottic airway devices: technology update [published correction appears in Med Devices (Auckl). 2018;11:27]. Med Devices (Auckl). 2017;10:189–205. PMID: 28860875.
- Shin HW, Yoo HN, Bae GE, et al. Comparison of oropharyngeal leak pressure and clinical performance of LMA ProSeal™ and i-gel® in adults: meta-analysis and systematic review. J Int Med Res. 2016;44:405–418. PMID: 27009026.
- Zhang J, Drakeford PA, Ng V, et al. Ventilatory performance of AMBU® AuraGain™ and LMA® Supreme™ in laparoscopic surgery: a randomised controlled trial. Anaesth Intensive Care. 2021;49:395–403. PMID: 34550812.
- Carron M, Veronese S, Gomiero W, et al. Hemodynamic and hormonal stress responses to endotracheal tube and ProSeal Laryngeal Mask Airway™ for laparoscopic gastric banding. Anesthesiology. 2012;117:309–320. PMID: 22614132.
- Brimacombe J. The advantages of the LMA over the tracheal tube or facemask: a meta-analysis. Can J Anaesth. 1995;42:1017–1023. PMID: 8590490.
- Tait AR, Pandit UA, Voepel-Lewis T, et al. Use of the laryngeal mask airway in children with upper respiratory tract infections: a comparison with endotracheal intubation. Anesth Analg. 1998;86:706–711. PMID: 9539588.
- Ye Q, Wu D, Fang W, et al. Comparison of gastric insufflation using LMA-supreme and I-gel versus tracheal intubation in laparoscopic gynecological surgery by ultrasound: a randomized observational trial. BMC Anesthesiol. 2020;20:136. PMID: 32493213.
- Park SK, Ko G, Choi GJ, et al. Comparison between supraglottic airway devices and endotracheal tubes in patients undergoing laparoscopic surgery: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e4598. PMID: 27537593.
- Higgins PP, Chung F, Mezei G. Postoperative sore throat after ambulatory surgery. Br J Anaesth. 2002;88:582–584. PMID: 12066737.
- Cohen MM, Cameron CB. Should you cancel the operation when a child has an upper respiratory tract infection? Anesth Analg. 1991;72:282–288. PMID: 1994755.
- Nevešćanin A, Vickov J, Elezović Baloević S, Pogorelić Z. Laryngeal mask airway versus tracheal intubation for laparoscopic hernia repair in children: analysis of respiratory complications. J Laparoendosc Adv Surg Tech A. 2020;30:76–80. PMID: 31613680.
- Loring SH, Behazin N, Novero A, et al. Respiratory mechanical effects of surgical pneumoperitoneum in humans. J Appl Physiol (1985). 2014;117:1074–1079. PMID: 25213641.
- Safran DB, Orlando R 3rd. Physiologic effects of pneumoperitoneum. Am J Surg. 1994;167:281–286. PMID: 8135322.
- Beleña JM, Ochoa EJ, Núñez M, et al. Role of laryngeal mask airway in laparoscopic cholecystectomy. World J Gastrointest Surg. 2015;7:319–325. PMID: 26649155.
- Maltby JR, Beriault MT, Watson NC, Fick GH. Gastric distension and ventilation during laparoscopic cholecystectomy: LMA-Classic vs. tracheal intubation. Can J Anaesth. 2000;47:622–626. PMID: 10930200.
- Maltby JR, Beriault MT, Watson NC, et al. LMA-Classic and LMA-ProSeal are effective alternatives to endotracheal intubation for gynecologic laparoscopy. Can J Anaesth. 2003;50:71–77. PMID: 12514155.
- Gordon J, Cooper RM, Parotto M. Supraglottic airway devices: indications, contraindications and management. Minerva Anestesiol. 2018;84:389–397. PMID: 29027772.
- Latorre F, Eberle B, Weiler N, et al. Laryngeal mask airway position and the risk of gastric insufflation. Anesth Analg. 1998;86:867–871. PMID: 9539617.
- Yoon SW, Kang H, Choi GJ, et al. Comparison of supraglottic airway devices in laparoscopic surgeries: a network meta-analysis. J Clin Anesth. 2019;55:52–66. PMID: 31871993.
- Bernardini A, Natalini G. Risk of pulmonary aspiration with laryngeal mask airway and tracheal tube: analysis on 65 712 procedures with positive pressure ventilation. Anaesthesia. 2009;64(12):1289-1294. PMID: 19860753.


 Issue PDF
Issue PDF PDF
PDF