INTRODUCTION
 In the second half of the last century, dopamine D2-receptor antagonists were a mainstay of the management of post-operative nausea and vomiting (PONV).1 However, at the start of the 21st century, they sharply declined in popularity, primarily as a result of growing safety concerns, not least of which was the imposition by the US Food and Drug Administration (FDA) of a black box warning on the most widely used agent in the class, droperidol.1
In the second half of the last century, dopamine D2-receptor antagonists were a mainstay of the management of post-operative nausea and vomiting (PONV).1 However, at the start of the 21st century, they sharply declined in popularity, primarily as a result of growing safety concerns, not least of which was the imposition by the US Food and Drug Administration (FDA) of a black box warning on the most widely used agent in the class, droperidol.1
Currently there is renewed interest in this class of medications related, in part, to the introduction of a new agent, amisulpride, which was approved by the FDA for the prevention and treatment of PONV in 2020 and is the only approved agent for rescue treatment after failed prophylaxis.
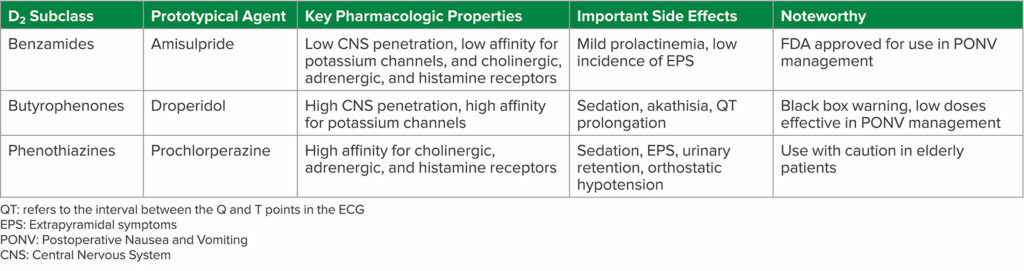
Re-evaluation of the evidence around D2-antagonists suggests they are not interchangeable in terms of either safety or efficacy, as this is an unusually heterogeneous class of drugs. There are at least three distinct structural sub-classes—substituted benzamides, butyrophenones and phenothiazines—with a wide range of pharmacologic properties and side effect profiles (Table 1).
Table 1: D2 Subclass of Antiemetics
SAFETY
D2-antagonists originally used as antiemetics were classical neuroleptics and first-generation antipsychotics (FGA).2 Central nervous system (CNS) penetration by D2-antagonist antiemetics results in a wide range of effects. Sedation and neuropsychiatric effects such as dysphoria or cognitive impairment can occur.2 Extrapyramidal symptoms (EPS) include tardive dyskinesia, dystonia, and akathisia.2 Neuroleptic malignant syndrome (NMS) presents with fever, mental status changes, muscle rigidity, and autonomic instability, and antagonism of D2-receptors in the pituitary results in hyperprolactinemia.2 In addition, binding to potassium ion channels can result in QT prolongation and torsade de pointes.2 Amisulpride is an “atypical” or second-generation antipsychotic with less brain penetration than FGAs,3 resulting in a lower incidence of these adverse effects.2
Although some of the side effects of D2-antagonists are dose-dependent, toxicity exists, and evidence is lacking on the impact of dose reduction on efficacy. Moreover, despite a reduction in frequency, adverse reactions like tardive dyskinesia, dysphoria, or torsade de pointes can have a high impact on patients. The crude incidence rate may not properly reflect the clinical burden. Therefore, it is essential to understand the relative risks of the available D2-antagonists in order for providers to make optimal prescribing decisions.
BENZAMIDES
Amisulpride is a substituted benzamide D2-antagonist and 5-HT2B and 5-HT7A serotonin antagonist with low blood-brain barrier penetration and lower affinity for adrenergic, histamine, and cholinergic receptors, resulting in a lower incidence of anticholinergic and sedative effects.4 Amisulpride also has preferential binding in the limbic system, resulting in a lower incidence of EPS.4 A 2020 Cochrane network meta-analysis reported that amisulpride had a comparable incidence of adverse events as compared to placebo.5 Elevated prolactin levels from amisulpride do not exceed the norm for nonpregnant women,6 and amisulpride does not meaningfully prolong the QT interval at doses used for PONV management due to its weaker affinity for potassium channels.7 Recent studies have shown that amisulpride is effective in both preventing PONV8 and as rescue treatment for PONV.9 Another benzamide D2-antagonist is metoclopramide, which is a weak D2 and 5-HT3 antagonist with dose dependent side effects that include sedation, EPS, and GI upset due to stimulation of gastric smooth muscle cells.10 In the literature, metoclopramide may be useful in institutions where other D2-antagonists are not available, but otherwise it may not be very efficacious in the management of PONV.1
BUTYROPHENONES
Droperidol is a butyrophenone D2-antagonist and was used as a first-line agent for PONV prophylaxis in low doses in the past.1 It produces sedation, dysphoria, anxiety, akathisia, and, most notably, QT prolongation.11 Although instances of sudden cardiac death led to an FDA black box warning in 2001 and a significant decline in its use,1 the 2020 Cochrane network meta-analysis reported that antiemetic doses of droperidol had a comparable incidence of adverse events to placebo.5 Following the FDA black box warning on droperidol, there was increased interest in haloperidol, another butyrophenone, in the management of PONV.1 Haloperidol produces sedation, EPS, neurotoxicity, and QT prolongation, and in 2007, the FDA updated labelling to warn providers that torsades de pointes and QT prolongation have been observed in patients receiving haloperidol, especially when administered via IV or in higher doses than recommended, emphasizing that haloperidol is not approved for IV administration for PONV treatment.12 However, evidence suggests that low doses of IV haloperidol appear to be safe and effective when given as a single dose for PONV prophylaxis.12
PHENOTHIAZINES
Prochlorperazine is the most commonly used phenothiazine D2-antagonist and FGA, producing sedation, EPS, anticholinergic effects (such as anorexia, blurred vision, constipation, dry mucosa, and urinary retention), antiadrenergic effects leading to orthostatic hypotension, and a decrease in the seizure threshold.13 Promethazine is another phenothiazine D2-antagonist and antihistamine that produces sedation, but IV formulations are irritating and corrosive, causing severe tissue damage upon extravasation from a vein.14
D2 ANTAGONIST SIDE EFFECTS
D2-antagonists can have notable drug interactions and are not recommended in patients with prolonged QT syndrome or taking drugs that prolong the QT interval, given the risk of further prolongation.15 Ondansetron, a commonly used antiemetic, can also prolong the QT interval, but the QT prolongation induced by the combination of ondansetron and droperidol is not different from that induced by each drug alone.1 D2-antagonists can potentiate QT prolongation in patients taking drugs that reduce heart rate or induce hypokalemia, and combining D2-antagonists with antipsychotics creates an additive risk for tardive dyskinesia and NMS.15 In addition, patients taking dopamine agonists such as levodopa for Parkinson’s or cabergoline for hyperprolactinemia should avoid D2 antagonists.15 Finally, D2-antagonists should not be given with monoamine oxidase (MAO) inhibitors, as norepinephrine is broken down by MAO, and D2-antagonism creates an accumulation of norepinephrine, leading to an exaggerated end-organ response.16
Best practices for postoperative brain health suggest that D2-antagonist antiemetics should be used with caution or avoided in patients over 65 as they can produce central anticholinergic effects (phenothiazines), EPS (benzamides), and tardive dyskinesia, delirium, and NMS (butyrophenones).17 Also, elderly patients with dementia may have an increased risk of cerebrovascular accident and an increased rate of cognitive decline and mortality with these medications.17 Similar to adult patients, pediatric patients may experience EPS and QT prolongation with D2-antagonists.18
PONV AND CLINICAL PRACTICE GUIDELINES
PONV contributes to prolonged postanesthesia care unit (PACU) stay, unanticipated hospital admission, and increased health care costs.1 The fourth consensus guidelines for the management of PONV published in 2020 outline identification of high-risk patients, managing baseline PONV risks, choices for prophylaxis, and rescue treatments of PONV.1 Two important conclusions from the guidelines should be highlighted here. Prevention of PONV should be considered an integral aspect of anesthesia, and therefore, patients with even one or two risk factors for PONV should receive multimodal PONV prophylaxis.1 In addition, PONV treatment should consist of an antiemetic from a pharmacologic class that is different from the prophylactic drug initially given,1 as there is no benefit of redosing ondansetron, despite its common practice.1
Various D2-antagonists have been shown to play a beneficial role in both PONV prophylaxis and treatment in the literature. Numerous randomized controlled trials and retrospective database analyses demonstrate that combination regimens of non D2-antagonist antiemetics with various older D2-antagonists such as droperidol, haloperidol, and promethazine, are more effective than either agent alone.5,19-21 However, the use of these agents has declined.19 To date, amisulpride has been evaluated for the management of PONV in six clinical trials.19,20 While five of the trials evaluated monotherapy and demonstrated amisulpride is superior to placebo in the prevention and treatment of PONV,6,8,22,23 Kranke et al. demonstrated that the combination of amisulpride with ondansetron or dexamethasone was more effective than ondansetron or dexamethasone alone in reducing PONV and for rescue PONV treatment.8
CONCLUSION
Multimodal PONV prevention and management is critical, especially in enhanced recovery after surgery (ERAS) pathways, patients undergoing ambulatory surgery, and treatment of high-risk patients who have increased acuity and fragility. D2-antagonists can play an effective role given the evidence in the literature, but they also have a wide range of side effects, limiting their use.24 However, amisulpride is a D2-antagonist with a favorable safety profile, as well as FDA approval for use in the prevention and management of PONV. Therefore, more studies are warranted to compare amisulpride to other single agent antiemetics and its use in combination therapy, as well as cost-benefit analyses.
Connie Chung, MD, is an assistant professor in the Department of Anesthesiology, University of Southern California Keck School of Medicine, Los Angeles, CA.
Joseph W. Szokol, MD, JD, MBA, is a professor in the Department of Anesthesiology, University of Southern California Keck School of Medicine, Los Angeles, CA.
The authors have no conflicts of interest.
REFERENCES
- Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131:411–448. PMID: 32467512.
- Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–777. PMID: 28721057.
- Natesan S, Reckless GE, Barlow KB, et al. Amisulpride the ‘atypical’ atypical antipsychotic—comparison to haloperidol, risperidone and clozapine. Schizophr Res. 2008;105:224–35. PMID: 18710798.
- Smyla N, Koch T, Eberhart LH, Gehling M. An overview of intravenous amisulpride as a new therapeutic option for the prophylaxis and treatment of postoperative nausea and vomiting. Expert Opin Pharmacother. 2020;21:517–522 PMID: 31971450.
- Weibel S, Rucker G, Eberhart LH, et. al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis. Cochrane Database Syst Rev.2020;10:CD012859. PMID: 33170514.
- Gan TJ, Kranke P, Minkowitz HS, et al. Intravenous amisulpride for the prevention of postoperative nausea and vomiting: two concurrent, randomized, double-blind, placebo-controlled trials. Anesthesiology. 2017;126:268–275. PMID: 27902493.
- Fox GM, Albayaty M, Walker JL, et al. Intravenous amisulpride does not meaningfully prolong the QTc interval at doses effective for the management of postoperative nausea and vomiting. Anesth Analg. 2021;132:150–159. PMID: 31913911.
- Kranke P, Bergese SD, Minkowitz HS, et al. Amisulpride prevents postoperative nausea and vomiting in patients at high risk: a randomized, double-blind, placebo-controlled trial. Anesthesiology. 2018;128:1099–1106. PMID: 29543631.
- Habib AS, Kranke P, Bergese SD, et al. Amisulpride for the rescue treatment of postoperative nausea or vomiting in patients failing prophylaxis: a randomized, placebo-controlled phase III trial. Anesthesiology. 2019;130:203–212. PMID: 30475232.
- Harrington RA, Hamilton CW, Brogden RN, et al. Metoclopramide. An updated review of its pharmacological properties and clinical use. Drugs. 1983;25:451–494. PMID: 6345129.
- Lim BS, Pavy TJ, Lumsden G. The antiemetic and dysphoric effects of droperidol in the day surgery patient. Anaesth Intensive Care. 1999;27:371–374. PMID: 10470391.
- Habib AS, Gan TJ. Haloperidol for postoperative nausea and vomiting: are we reinventing the wheel? Anesth Analg. 2008;106:1343–1345. PMID: 18420842.
- Din L, Preuss CV. Prochlorperazine. In: StatPearls. Treasure Island (FL) 2022. PMID: 30725768.
- Southard BT, Al Khalili Y. Promethazine. In: StatPearls. Treasure Island (FL) 2022. PMID: 31335081.
- Chokhawala K, Stevens L. Antipsychotic Medications. In: StatPearls. Treasure Island (FL) 2022. PMID: 30137788.
- Sub Laban T, Saadabadi A. Monoamine Oxidase Inhibitors (MAOI). In: StatPearls. Treasure Island (FL) 2022. PMID: 30969670.
- Berger M, Schenning KJ, Brown CH 4th, et al. Best practices for postoperative brain health: recommendations from the fifth International Perioperative Neurotoxicity Working Group. Anesth Analg. 2018;127:1406–1413. PMID: 30303868.
- Kovac AL. Management of postoperative nausea and vomiting in children. Paediatr Drugs. 2007;9:47–69. PMID: 17291136.
- Haber SL, Graybill A, Minasian A. Amisulpride: a new drug for management of postoperative nausea and vomiting. Ann Pharmacother. 2021;55:1276–1282. PMID: 33412897.
- Habib AS, Gan TJ. The effectiveness of rescue antiemetics after failure of prophylaxis with ondansetron or droperidol: a preliminary report. J Clin Anesth. 2005;17:62–65. PMID: 15721732.
- Habib AS, Reuveni J, Taguchi A, et al. A comparison of ondansetron with promethazine for treating postoperative nausea and vomiting in patients who received prophylaxis with ondansetron: a retrospective database analysis. Anesth Analg. 2007;104:548–551. PMID: 17312206.
- Kranke P, Eberhart L, Motsch J, et al. I.V. APD421 (amisulpride) prevents postoperative nausea and vomiting: a randomized, double-blind, placebo-controlled, multicentre trial. Br J Anaesth. 2013;111:938–945. PMID: 23872464.
- Candiotti KA, Kranke P, Bergese SD, et al. Randomized, double-blind, placebo-controlled study of intravenous amisulpride as treatment of established postoperative nausea and vomiting in patients who have had no prior prophylaxis. Anesth Analg. 2019;128:1098–1105. PMID: 31094774.
- Tan HS, Dewinter G, Habib AS. The next generation of antiemetics for the management of postoperative nausea and vomiting. Best Pract Res Clin Anaesthesiol. 2020;34:759–769. PMID: 33288125.


 Issue PDF
Issue PDF PDF
PDF