Introduction
High-Flow Nasal Oxygen (HFNO) administration is a relatively new technique that is used in the intensive care unit (ICU), and increasingly in the operating room (OR). HFNO has become popular in the ICU for management of patients with acute hypoxemic respiratory failure when attempting to avoid intubation or to help after extubation. In some anesthesia contexts, HFNO has been referred to as THRIVE—an abbreviation for Transnasal Humidified Rapid-Insufflation Ventilatory Exchange. Active research is ongoing as to the wider applications of HFNO. This brief current review will discuss the underlying mechanisms of HFNO, its potential use in clinical anesthesia practice, and the risks and benefits of such use. It focuses on the use of HFNO in adult patients, not children.
HFNO Mechanism and Component Parts
There is a marked difference between oxygen administration with standard low flow nasal cannulae and HFNO. When patients are administered low flow nasal O2, the oxygen flow rates are typically between 2–10 liters/minute (L/M). Spontaneously breathing patients typically have an inspiratory flow rate (IFR) of 20–40 L/M. Once the IFR exceeds the flow of O2 coming from the nasal cannulae, room air will be entrained which dilutes the FiO2. The effective delivered oxygen concentration (which reaches the lungs) is usually 25–30%, if a patient is receiving 2–4 L/M of nasal O2.
In contrast, HFNO uses oxygen flows of 50–100 L/M. With this technique, the high flows delivered via the specially designed nasal cannulae now exceed the patient’s IFR. Therefore, there is little entrainment of room air which allows the delivery of a high FiO2 (95–100%).
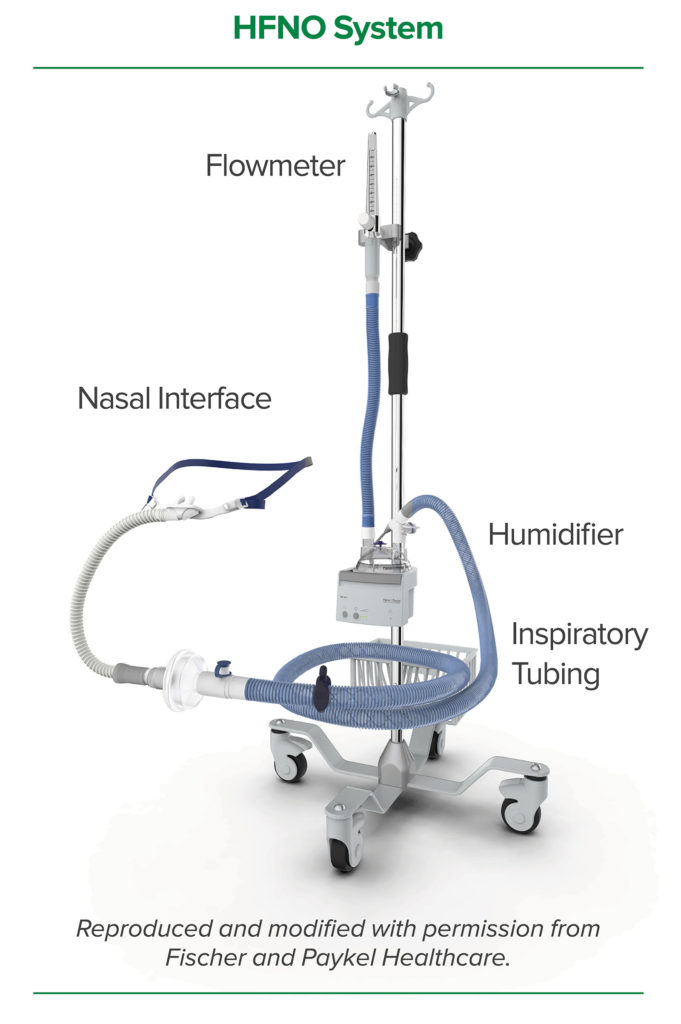
The components of a HFNO system are
- An electrically powered high-pressure oxygen/air supply (ideally with a blender to blend air into the gas flow to reduce the FiO2 if needed)
- A flowmeter capable of flows of up to 100 liters per minute
- A humidifier capable of fully humidifying the inspired oxygen/air mixture
- Wide bore tubing to deliver gas from the gas supply to the nasal cannulae
- Specialized wide bore nasal cannulae, which convey the oxygen/air blend from the gas tubing to the patient’s nose.
Beneficial physiologic effects of HFNO
HFNO has a number of beneficial effects not provided by standard nasal cannula. At high flow rates, it can provide continuous positive airway pressure (CPAP), washes out CO2 from the respiratory dead space, and assists the process of oxygen diffusion into the alveoli (replacing oxygen which has been absorbed).1-3 In addition, it can reduce the work of breathing and reduce airway resistance.4
HFNO is capable of delivering very high gas flows with high FiO2 or oxygen/air blends to anesthetized, sedated, or awake patients. Depending on the physiology of the patient, HFNO may have benefits for clinical anesthetic management, but it is important to recognize that use of HFNO has its own inherent risks. Several applications of HFNO are described below, each with its potential benefits and risks.
Clinical applications of HFNO with specific benefits and risks:
- Improving preoxygenation before induction of general anesthesia (GA)
Preoxygenation using HFNO can be a good alternative to standard preoxygenation, which is usually performed with an FiO2 of 1.0 delivered via a closed anesthesia breathing circuit and an appropriately fitted face mask.5,6 HFNO is well-tolerated by awake patients at flow rates of 30–40 liters per minute, provides effective preoxygenation without the use of a facemask, and provides ongoing CPAP, which reduces pulmonary shunting. In addition, the preoxygenation with HFNO can be continued into the peri-intubation period of oxygenation.
- Providing ongoing oxygenation and CO2 removal for patients during intubation
The use of HFNO during the intubation process can extend the time interval until critical desaturation through the delivery of apneic oxygenation. This is especially attractive during a Rapid Sequence Intubation (RSI), where mask ventilation is not performed prior to intubation.1 Another benefit of providing HFNO during intubation is that CO2 accumulation is limited, especially in the first 20 minutes,1 due to the effect of HFNO washing out CO2. This effect can be especially useful for difficult intubations which may require more time to secure the airway. One important aspect of HFNO use to be aware of in this context is that the patient is not receiving a volatile anesthetic. Thus, supplemental intravenous anesthesia should be provided during this time period. In addition, if the time interval of HFNO is prolonged (more than 20 minutes), then methods for providing additional ventilation and CO2 removal are required.1 Twenty minutes is a guideline, and will vary depending on the physiology of the patient.
- Providing effective oxygenation during awake oral or nasal fiberoptic or videoscopic intubation
With HFNO use, patients undergoing awake orotracheal intubation have improved O2 delivery and receive some CPAP while the oral airway is unobstructed for intubation. Surprisingly, CPAP is delivered even if the mouth remains open although it is less effective than when the mouth is closed.2 Topical anesthetic preparation and subsequent fiberoptic nasal intubation can be achieved by working around the nasal cannula, when a nasal intubation is desired. However, the nasal cannula on the side of intubation must be removed prior to nasotracheal tube placement. HFNO may also benefit patients with partially obstructed airways undergoing awake intubation because of its ability to reduce both the work of breathing and airway resistance.
- Providing respiratory support after extubation
Patients who have recently been extubated and require partial respiratory support to maintain oxygenation/ventilation may benefit from HFNO.2,3 HFNO provides a well-tolerated form of CPAP (at the level of 3–4 cm H2O with the mouth open) in addition to oxygen delivery. It does not cover the mouth so patients can talk while using HFNO. It is arguably a simpler technology to set up and use compared to many CPAP/ventilator machines and masks. However, one identifiable risk is that casual removal of the HFNO (by providers assuming it is “standard low-flow nasal oxygen”) may result in an acute hypoxemia and respiratory insufficiency.
- Providing oxygenation, reducing work of breathing, and facilitating CO2 elimination for use during surgical procedures
HFNO can be beneficial for sedated or even anesthetized (with IV medications) patients who are breathing spontaneously and even with some procedures requiring periods of apnea.1,7 The benefit is that adequate oxygenation and ventilation can be provided, and yet the oral aperture, larynx, face, neck and all other areas apart from the nose are free to be operated upon. This could include cases with a partially obstructed airway, such as patients undergoing a tracheostomy.
Contraindications to and risks of HFNO
Suggested relative contraindications to HFNO are
- Partial nasal obstruction
- Disrupted airway, e.g., laryngeal fracture, mucosal tear, or tracheal rupture
- Need for laser or diathermy (electrosurgery) in proximity to the administration of HFNO which increases fire risk. (This changes to an absolute contraindication under many circumstances that involve an FiO2 of >30%.)
- Contagious pulmonary infections, such as tuberculosis
- Nasal infection resulting in pulmonary seeding with HFNO use is a theoretical concern. However, there is no evidence to date that demonstrates pulmonary seeding with HFNO
- Contraindications to high concentrations of oxygen (e.g., prior bleomycin chemotherapy)
- Inability to tolerate hypercarbia if HFNO is used with prolonged apnea (e.g., patients with sickle cell anemia, pulmonary hypertension, intracranial hypertension, and some forms of congenital heart disease)
- Children under the age of 16. Cases of air-leak syndrome (i.e., pneumothorax) have been reported with HFNO use in children below the age of 16.8 These were serious events and suggest that research and expert guidance is warranted to determine the safe use of HFNO in children.
Absolute contraindications to HFNO are
- Use of alcohol-based skin preparation solutions in combination with HFNO, which increases the fire risk
- Known or suspected skull base fractures, CSF leaks, or any other communication from the nasal to the intracranial space
- Significant pneumothorax which has not been treated with a chest tube. The CPAP effect may expand the pneumothorax.9
- Complete nasal obstruction
- Active epistaxis or recent functional endoscopic sinus surgery (FESS).
The application of a tightly sealed mask on top of HFNO cannulae could potentially create too much pressure if the anesthetic machine APL valve is closed, which is why the manufacturers of one HFNO device advise against this (The Fisher and Paykel Optiflow. Fisher and Paykel Healthcare Limited, Panmure, Auckland 1741, New Zealand).
Some additional scenarios posing potential risks with HFNO use
The authors are not advocating for or against the use of HFNO for these scenarios. We are simply pointing out some of the more important considerations in the risk/benefit analysis of this approach, which is especially important as it is already part of existing practice for some clinicians.
- HFNO delivery under the surgical drapes
A specific risk apart from those mentioned under contraindications is the potential fire risk when HFNO is delivered under surgical drapes. The oxygen-rich environment created with high FiO2 HFNO only needs a trigger (such as diathermy) to ignite, while drapes and swabs in the surgical field can serve as a potential fuel source.10 The risk with this kind of oxygen “pollution” has been seen in videos of mock ignition.11 Important factors impacting the fire ignition risk include duration of HFNO use, adhesion of drapes to create barriers to O2 flow, flow rate, FiO2 of HFNO, and OR room air exchange rates. If HFNO is used in this context, particular care must be taken with all three parts of the fire ignition triad—namely the HFNO flow rate and FiO2, the fuel sources, and the use of ignition devices. The FiO2 can be adjusted (down to room air) with an air/oxygen gas blender. This will reduce the fire risk, while maintaining some benefits of HFNO to patient care.
- Performing an emergent awake tracheostomy in patients with partial airway obstruction
Performing an emergent awake tracheostomy may be required for patients who have severe partial airway obstruction.12,13 HFNO has been employed for performing an emergent awake tracheostomy in this context, which may also include the use of sedation.14 The benefits of the HFNO-with-sedation technique include improved oxygenation and time to desaturation, decreased work of breathing, and potentially a more cooperative patient. The specific risks include the potential loss of the airway and hypoxia. Furthermore, depending on the amount of FiO2 used with HFNO, the risk of airway fire may be increased compared with traditional methods of oxygen delivery.
- Elective airway surgery
HFNO may be useful during elective surgical procedures such as on the airway (e.g., microlaryngoscopy) where sedation or IV GA is often used.1,6 In this setting, HFNO can be used with spontaneous ventilation. If periods of apnea are required, intermittent bag mask ventilation can be used to address the slow build-up of CO2. The benefits of HFNO in this setting include improved oxygenation (even with prolonged apnea), decreased work of breathing, and even some CO2 removal which results from HFNO washout.The risk of HFNO use for elective airway surgery is oxygen contamination of the operative field, which increases the fire risk both at the surgical site and the upper half of the patient covered by surgical drapes. This risk is especially relevant where lasers or diathermy (ESU) are used (Figure 1).Providers must balance the benefits of improved oxygenation and ventilation provided by HFNO with the potential fire risk. Modern jet ventilators that are used during microlaryngoscopy15 have specific safety features to lower the FiO2 when a laser will be used. Jet ventilation frequently entrains room air, which will decrease the FiO2. However, the resultant FiO2 is variable and, therefore, frequently unknown to the anesthesia professional. Part of the risk profile of HFNO is that it is often configured for use only with 100% oxygen, and there may be no way to reduce the FiO2.The manufacturers of one version of a commonly used HFNO system—The Fisher and Paykel Optiflow (Fisher and Paykel Healthcare Limited, Panmure, Auckland 1741, New Zealand)—clearly state: “To avoid burns…Do not use the system near any ignition source, including electrosurgery, electrocautery, or laser surgery instruments. Exposure to oxygen increases the risk of fire.” The medical warning is clear. In addition, this statement will likely be part of any medico-legal action if a fire should occur while using HFNO. This caution, however, has not stopped the use of HFNO in clinical practice and research into the use of HFNO during laser laryngeal surgery.7 An OR fire case involving HFNO has already been reported.16
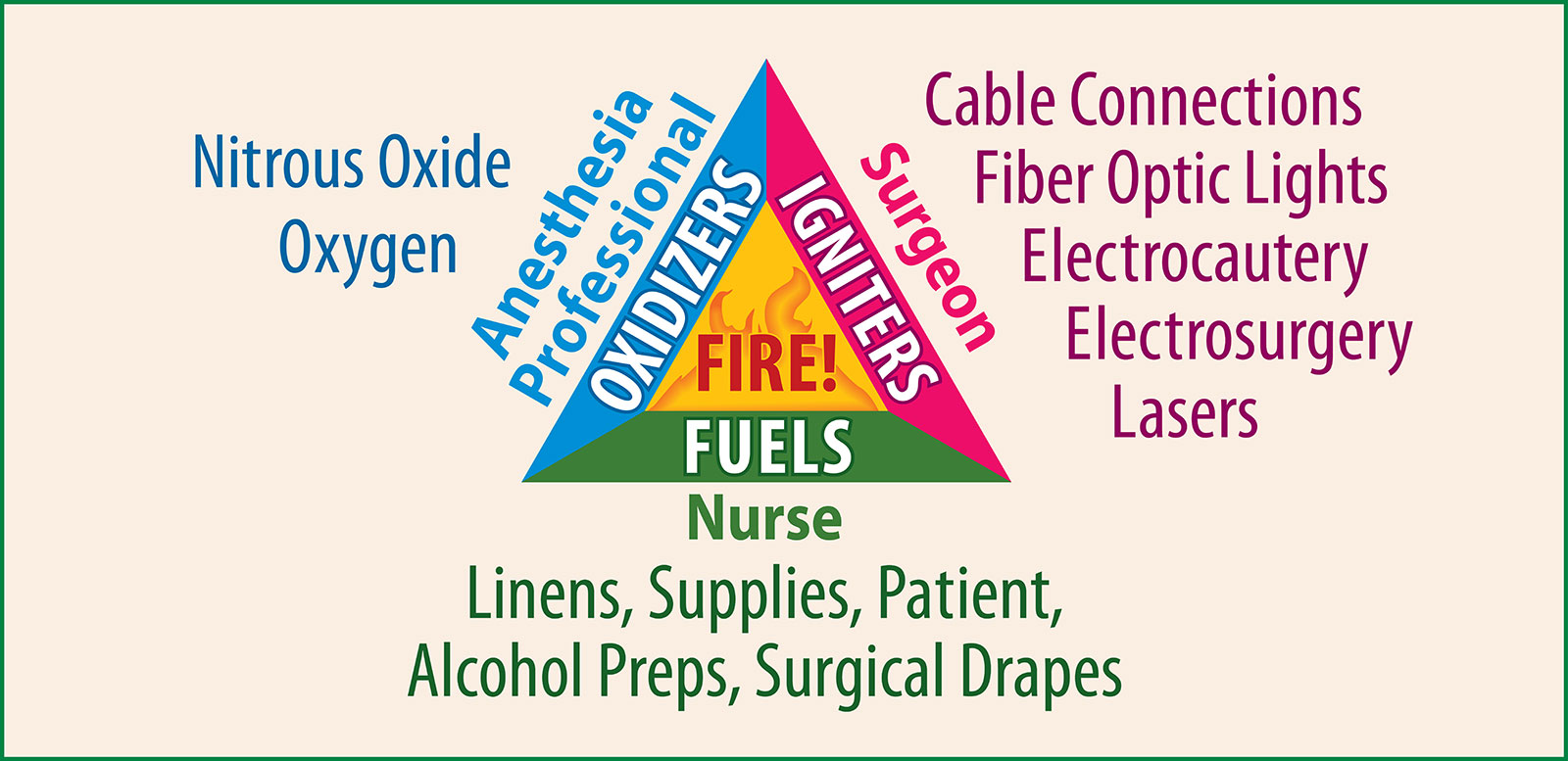
Figure 1. Illustrates the three elements needed to initiate a fire: oxygen, fuel, ignition source.
Reproduced from the APSF 2014. Fire Safety Prevention Poster https://www.apsf.org/videos/or-fire-safety-video/ Accessed on August 20, 2018.
Guiding considerations to assess fire risk of HFNO use:
- Some authors have distinguished between accidental flash flames and spreading flames the latter of which causes more damage (burns).17 Reports of HFNO fires have not been reported frequently enough to make a judgement about the kinds of flames produced and more research is indicated.
- We do not have a clear idea of overall ignition frequency with cases performed under alternative ventilation techniques; thus, comparative fire risk is unknown.
- Using an oxygen/air blender to reduce the FiO2 with HFNO should help to reduce the risk of fire.
- HFNO is a new technology and the reports of two fires described at this early stage of adoption may herald more fires in the future as HFNO gains in popularity. *16 Practitioners must exercise extreme care to reduce the fire risk.
- To date, no patient harm has been reported.
- The oxygen “pollution” around the head and neck area from HFNO use has not been comprehensively studied. An APSF video which focuses on intraoperative fire risk indicates that any oxygen concentration greater than 30% in the head and neck area creates an increased fire risk, especially for procedures in that area.18
Future Considerations
It is likely that an increasing number of anesthesia professionals will utilize HFNO in the operating room. One obstacle is that the HFNO equipment must be brought into the operating room and assembled every time it is used. In the future, HFNO could be designed to directly connect to the anesthetic workstation for easier use. Due to regulatory and manufacturing limitations, however, it is unlikely that such modifications to incorporate HFNO apparatus will soon be available. Anesthesia professionals should encourage manufacturers to recognize these issues and work towards adding this feature to the next generation of machines.
Conclusions
HFNO is a novel system of respiratory support, which allows delivery of oxygenation at variable concentrations, reduces the work of breathing, provides CPAP, and assists in CO2 removal. While it has a number of potential uses in anesthetic and perioperative practice, it also has definite relative and absolute contraindications. The potential risks of harm with HFNO use are probably underappreciated. Many questions regarding benefits and safety in specific clinical contexts remain. Before using HFNO, education and insight into its use is highly recommended.
Dr. Cooper is presently an anaesthesia consultant at the Green Lane Dept. of Cardiothoracic and ORL Anaesthesia, Auckland City Hospital, Auckland, New Zealand.
Dr. Griffiths is an anaesthesia consultant at the Green Lane Dept of Cardiothoracic and ORL Anaesthesia, Auckland City Hospital, Auckland , New Zealand.
Dr. Ehrenwerth is professor emeritus, Yale University School of Medicine, New Haven, CT USA.
Both Dr. Cooper and Griffiths have assisted with clinical research in HFNO for Fisher and Paykel Ltd, but have received no funds or other compensation from this entity. Dr. Ehrenwerth reports no conflicts of interest.
References
- Patel A, Nouraei SA. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE): a physiological method of increasing apnea time in patients with difficult airways. Anaesthesia 2015;70:323–329.
- Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care 2007;20:126–131.
- Parke R, McGuiness S, Eccleston M, et al. High-flow therapy delivers low level positive airway pressure. Br J Anaesth 2009;103:886–890.
- Dysart K, Miller TL, Wolfson MR, et al. Research in high flow therapy: Mechanisms of action. Resp Med 2009;103:1400–1405.
- Simon M, Wachs C, Braune S, et al. High-flow nasal cannula versus bag-valve-mask for preoxygenation before intubation in subjects with hypoxemic respiratory failure. Respir Care 2016;61:1160–1167.
- Nimmagadda U, Ramez Salem M, Crystal GJ. Preoxygenation: Physiological basis, benefits, and potential risks. Anesth Analg 2017;124:507–517.
- Booth AWG, Vidhani K, Lee PK, et al. SponTaneous Respiration using IntraVEnous anaesthesia and Hi-flow nasal oxygen (STRIVE Hi) maintains oxygenation and airway patency during management of the obstructed airway: an observational study. Br J Anaesth 2017;118:444–451.
- Hegde S, Prodhan P. Serious air leak syndrome complicating high-flow nasal cannula therapy: a report of 3 cases. Pediatrics 2013;131:e939–44.
- Wiersema Ubbo F. Noninvasive respiratory support. In: Sidebotham D, McKee A, Gillham M, Levy JH, editors. Cardiothoracic critical care. First ed. Philadelphia: Butterworth, Heinemann, Elsevier; 2007.
- Ehrenwerth J. Electrical and fire safety. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, Ortega R, Sharar SR, and Holt NF, editors. Clinical anesthesia. 8th ed. Philadelphia: Wolters Kluwer; 2017.
- Cooper JO. Anesthesiology thermal injury. You Tube https://www.youtube.com/watch?v=FjA3dEyutt4. Accessed on July 1, 2018.
- Fang C, Friedman R, White PE, et al. Emergent awake tracheostomy—the five-year experience at an urban tertiary care center. Laryngoscope 2015;125:2476–9.
- Mason RA, Fielder CP. The obstructed airway in head and neck surgery. Anaesthesia 1999;54:625–628.
- Desai N, Fowler A. Use of transnasal humidified rapid-insufflation ventilatory exchange for emergent surgical tracheostomy: a case report. A A Case Rep 2017;9:268–270.
- Monsoon ventilator, Acutronic Medical Systems AG. Fabrik im Schiffli, 8816 Hirzel, Switzerland.
- Onwochei D, El-Boghdadly K, Oakley R, et al. Intra-oral ignition of monopolar diathermy during transnasal humidified rapid-insufflation ventilatory exchange (THRIVE). Anaesthesia 2017;72:781–783.
- Jones E, Overbey D, Chapman BC, et al. Operating Room Fires and Surgical Skin Preparation. J Am Coll Surg 2017;225:160–165.
- OR Fire Safety Video. https://www.apsf.org/videos/or-fire-safety-video/ Accessed on July 1, 2018.


 Issue PDF
Issue PDF