Defective Central Venous Catheter Introducer Needle
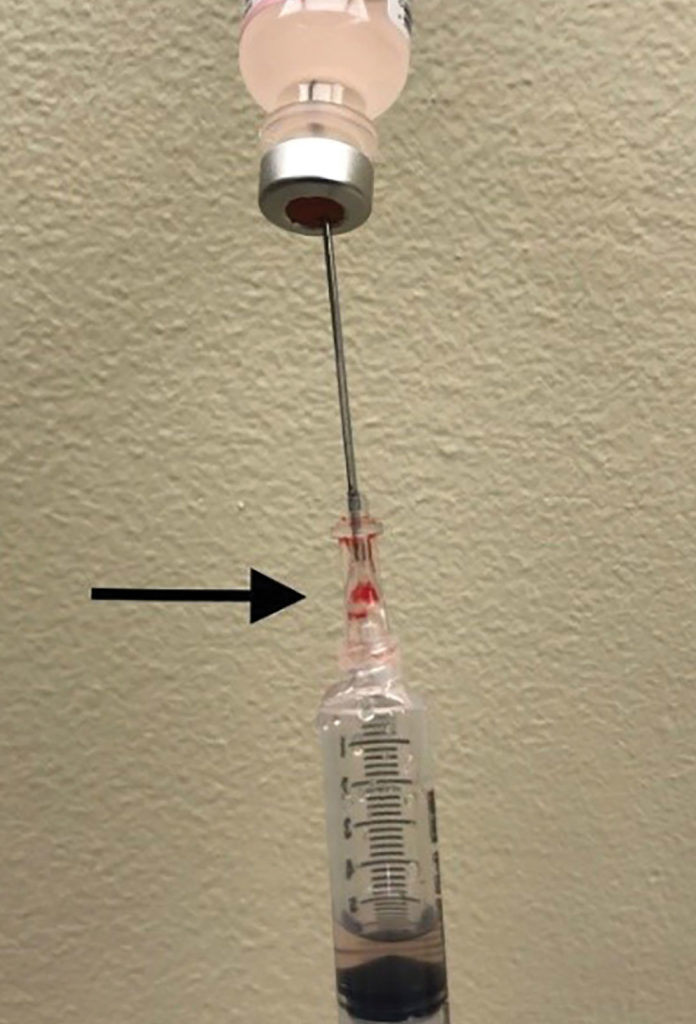
Figure 1. This figure depicts air aspirated into syringe instead of saline from vial due to a defect in the hub of the introducer needle. Arrow points to hub defect location.
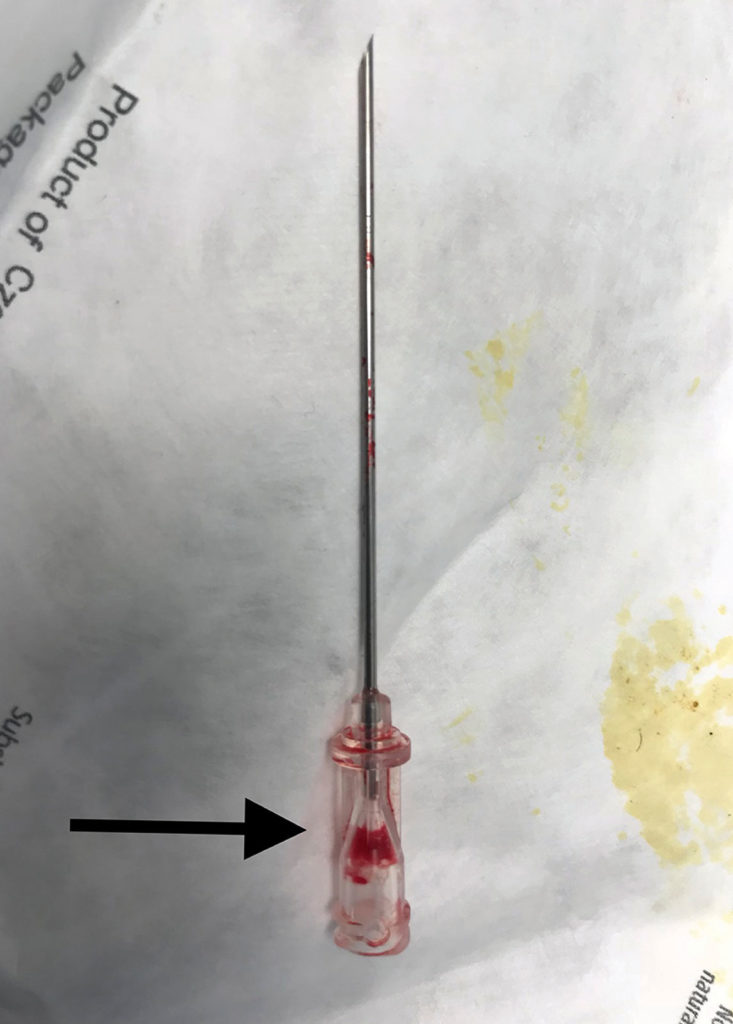
Figure 2. This figure depicts the 18 gauge introducer needle with a defect in the hub resulting in an inability to aspirate fluid. Arrow points to hub defect location.
We are writing to describe an incident we experienced involving a central line kit that has implications for patient safety. The case involved a 70-year-old morbidly obese patient scheduled for emergency craniotomy for intracranial hemorrhage. Due to lack of intravenous access and need for vasopressors, an ARROWgard Blue PLUS® Two-Lumen AK-42802-CDC (Lot 13F17L0206) 8 French 16 cm length central venous catheter kit was utilized for attempted central venous access. Ultrasound guidance and aspiration of dark colored blood via a 22 gauge finder needle was utilized to confirm location of the internal jugular vein. However, no blood could be aspirated when utilizing the 18 gauge introducer needle attached to the 5 ml Arrow® Raulerson Spring-Wire Introduction Syringe included in the kit. The 5 ml syringe was replaced by another Luer-Slip syringe which was attached to the hub of the introducer needle, but still we were unable to aspirate blood despite ultrasound visualization of the needle tip in the vein. The procedure was aborted, and a saline aspiration test was performed using the 18 gauge introducer needle, however, only air could be aspirated (Figure 1). Close inspection of the introducer needle revealed air entry via a crack not visible to the naked eye at the plastic hub of the introducer needle (Figure 2). After the central line kit was replaced with a new kit, the internal jugular vein was accessed with one attempt. Postoperatively, a hematoma was noted at the central venous puncture site from multiple venous punctures due to the initial defective introducer needle. Ultrasound visualization of needle tip in the vein was helpful in early diagnosis of equipment malfunction. Although introducer needle defects are a rare event, routine saline aspiration testing of introducer needles for integrity prior to venous puncture should be considered in conjunction with ultrasound guidance. The authors are not aware of any other published reports of this type of needle equipment failure. Arrow International, Inc., a subsidiary of Teleflex, Inc., was informed of the incident, and the introducer needle was sent back to manufacturer for analysis.
Dr. Jackson Su is an associate professor in the Department of Anesthesiology and Perioperative Medicine at the University of Texas MD Anderson Cancer Center.
Dr. Allen Holmes is a clinical associate professor in the Department of Anesthesiology and Perioperative Medicine at the University of Texas MD Anderson Cancer Center.
The authors have no disclosures pertinent to this article.
Reply:
An incident was recently brought to my attention, in which there was an issue with our ARROWgard Blue PLUS® Two-Lumen, 8 French, 16 cm central venous catheter kit. I also was provided with a copy of a letter/case report submitted by Dr. Su, describing the incident in more detail, and it was appropriately forwarded to our Complaints Team at Teleflex. A sample of the product was eventually returned to us (including the introducer needle, syringe, and lidstock) for evaluation as well.
Cracks in the introducer needle hub may theoretically be caused by excessive stress or tension on the hub when it is attached to the syringe (either by the user/clinician during use of the device or during the manufacturing and/or assembly process). A device history record review was performed for this complaint type, and no relevant or significant manufacturing issues were identified. A risk evaluation assessment has been completed, and further investigation is being conducted to determine a root cause. Teleflex will continue to monitor this issue through post-market surveillance and implement any corrective and preventive actions if deemed necessary. Thus far, we have not noted an unacceptably high frequency of occurrence of this particular issue. However, close inspection of all the components in the procedural kit is always recommended when possible.
On behalf of Teleflex, I would like to thank you for bringing this to our attention. Teleflex takes patient safety very seriously, and I can assure you that—if necessary—every attempt will be made to mitigate any potential risks from our vascular access products in the future.
If you have any further questions or concerns, please feel free to contact me at any time.
Sincerely,
Chris Davlantes, MD, FACEP
Medical Director – Clinical and Medical Affairs
Teleflex Incorporated
The information provided is for safety-related educational purposes only, and does not constitute medical or legal advice. Individual or group responses are only commentary, provided for purposes of education or discussion, and are neither statements of advice nor the opinions of APSF. It is not the intention of APSF to provide specific medical or legal advice or to endorse any specific views or recommendations in response to the inquiries posted. In no event shall APSF be responsible or liable, directly or indirectly, for any damage or loss caused or alleged to be caused by or in connection with the reliance on any such information.




 Issue PDF
Issue PDF