The Institute for Safe Medication Practices (ismp.org) receives reports of medication safety issues from health care providers and regulatory agencies worldwide. On a biweekly basis these are collated and published in the ISMP Acute Care Medication Safety Alert Newsletter. In this current issue of the APSF Newsletter, we highlight two reports of interest to the anesthesia community that were recently published in the June 2018 ISMP Newsletters.
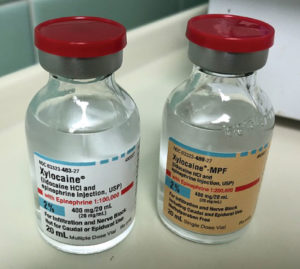
Figure 1. Demonstrates the similarities between multi-dose 2% lidocaine with epinephrine (on left), and single-dose without (on right), methylparaben. Modified and reproduced with permission from the ISMP.
The first report of note is a case of an anesthesia resident who intended to administer 2% lidocaine with epinephrine through an epidural in a patient scheduled to undergo a cesarean section. This local anesthetic solution had been removed from the anesthesia drug tray due to hospital shortages. However, the resident was able to access an automated dispensing cabinet (ADC) that contained the drug, which was listed on the ADC screen under the patient’s name. He retrieved a vial, but did not fully read the vial label before administering the drug through the epidural. The resident didn’t realize that it was a multiple-dose vial containing methylparaben, a preservative, and was labeled “Not for caudal or epidural use” 1 (Figure 1).
It has been standard practice to avoid preservative-containing solutions in this clinical situation, although toxicity from neuraxial administration of methylparaben preservative has not been clearly elucidated. Furthermore, multidose vials are no longer standard of care for any route of administration because of a higher risk of contamination than properly used single-use vials.1
The removal of preservative-containing local anesthetic products from the labor and delivery area and other areas where use of neuraxial local anesthetics is common (e.g., the operating room) should be considered. Health care professionals who work in these areas and those who stock these areas should be aware of the differences between these drugs.1
The second report stems from drug shortages and, in this case, involved look-alike lidocaine vials. Some hospitals have been forced to use products from more than one manufacturer, due to lidocaine shortages. The problem is that the 1% strength of lidocaine from one manufacturer (Figure 2) looks like the 2% concentration from another pharmaceutical company: the AuroMedics (East Windsor, NJ) 2% lidocaine looks like West-Ward Pharmaceutical’s (Cherry Hill, NJ) 1% lidocaine. The hospital that reported this hazard sent an email to pharmacy staff to make them aware of product similarities. ISMP recommends the use of barcode scanning in the pharmacy for drug verification.2
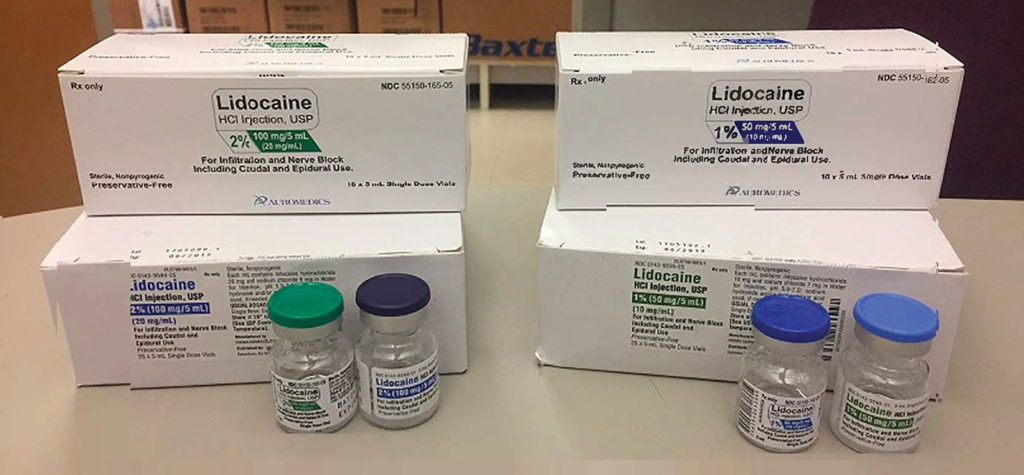
Figure 2. Depicts the similarities between two different concentrations of lidocaine made by two different companies.
If you have encountered medication errors, near misses or hazardous conditions you’d like others to learn about, please report them, in confidence, to ISMP (https://www.ismp.org/report-medication-error).
Dr. Ronald S. Litman, is an anesthesiologist in the Department of Anesthesiology and Critical Care Medicine at The Children’s Hospital of Philadelphia. He presently serves as Medical Director of the Institute for Safe Medication Practices.
William D. Ryan is an undergraduate student at Drexel University in Philadelphia, PA.
They have no conflicts of interest to disclose.
References
- Acute Care ISMP Safety Alert. Safety brief: avoid local anesthetics with preservatives for neuraxial use. ISMP Newsletter 2018;23:2.
- Acute Care ISMP Safety Alert. Safety brief: look-alike lidocaine vials. ISMP Newsletter 2018;23:3.


 Issue PDF
Issue PDF