Cardiovascular Implantable Electronic Device (CIED) technology continues to evolve and the global population of individuals with CIEDs is expanding. We present a focused update to the perioperative management of CIEDs since our last publication in 2020.
LEADLESS CIEDS
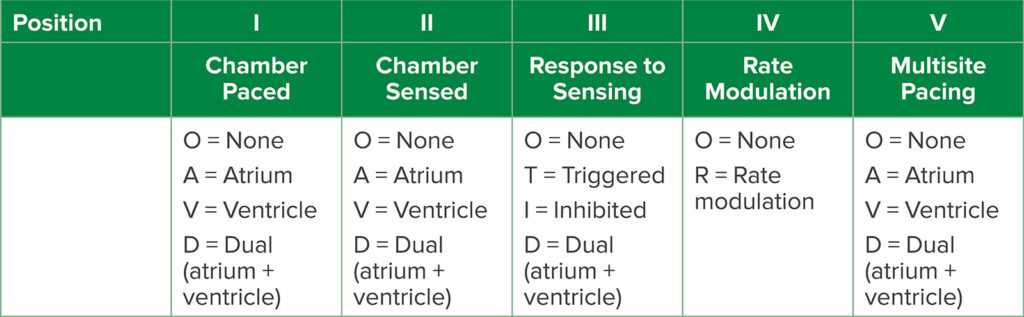
In our previous article in 2020, we introduced the Medtronic Micra™ leadless single-chamber ventricular pacemaker.1 This device is inserted via the femoral vein and implanted in the right ventricular endocardium. The interest in leadless devices is driven by vascular access challenges in some patients, such as those with end-stage renal disease and multiple previous hemodialysis lines, and those with congenital heart disease with abnormal vascular anatomy. In addition, transvenous CIEDs are susceptible to infection and lead fracture. In 2023, Medtronic received approval from the Food and Drug Administration (FDA) for its newest Micra pacemakers, with two different models: the Micra AV2 and the Micra VR2. Similar to the original Micra, the Micra VR2 is intended only for ventricular sensing and pacing for patients who have atrioventricular (AV) block or atrial fibrillation. The Micra AV2 is indicated for patients with AV block, but unlike the VR2, it can provide atrial sensing and synchronous ventricular pacing. The Micra AV2 uses an accelerometer to sense the atrium and is able to pace in a VDD mode (Table 1). The key point for anesthesia professionals is that the Medtronic Micra models are not responsive to magnet application. If the patient requires asynchronous (VOO) pacing due to a risk for electromagnetic interference, the pacemaker must be reprogrammed with the programmer device.
Table 1: Generic pacemaker codes from the North American Society of Pacing and Electrophysiology and British Pacing and Electrophysiology Group.2 Position refers to letter position in the pacemaker code (e.g., DDD, DOO, etc.).
The Abbott AVEIR™ VR, also a leadless device, was FDA-approved in 2022. The AVEIR VR has similar capabilities as the Micra; however, the AVEIR VR cannot perform AV sequential pacing (VDD) like the Micra AV. The AVEIR DR system, which was recently approved by the FDA, can perform dual chamber pacing. One advantage of the AVEIR devices is that they do respond to magnet placement. The magnet must be placed directly over the heart, and it will change the pacing mode to VOO at 100 beats per minute for five beats. If the battery is depleted, the magnet rate will then decrease to less than 100 depending on remaining battery life. Since the magnet response can be programmed off, anesthesia professionals should confirm magnet response prior to the start of the procedure by applying a magnet and observing the initial magnet rate of 100 for five beats.
MRI-CONDITIONAL DEVICES
CIED technology has evolved to include devices that are magnetic resonance (MR) conditional. This refers to a device that can be safely utilized in the MRI environment under specific conditions. CIEDs that do not meet MR conditional criteria are labeled MR nonconditional. There is the potential for patient morbidity and even mortality in the MRI environment related to CIED complications including generator movement, tissue heating, electromagnetic interference, and device reset. The practice advisory from the American Society of Anesthesiologists (ASA) from 2020 recommends that MR conditional devices should be interrogated prior to MRI and programmed to the magnetic resonance imaging mode.3 The device should be placed in an asynchronous pacing mode for pacemaker-dependent patients with suspension of anti-tachycardia therapy. Finally, the CIED should be interrogated after the MRI. The recommendations for MR nonconditional CIEDs are similar with respect to asynchronous pacing for pacemaker-dependent patients with suspension of anti-tachycardia therapy. The Heart Rhythm Society (HRS) 2017 guidelines also recommend that for MR nonconditional CIEDs, one should consider programming to non-pacing modes (e.g., ODO) or inhibiting modes (e.g., DDI) for patients that are not pacemaker -dependent.4 The HRS also states that it is reasonable for MR nonconditional CIEDs to have an MRI if there are no fractured, epicardial, or abandoned leads and MRI is the best diagnostic test to answer the clinical question. Perioperative guidelines for CIED care in the MRI environment also include EKG and pulse oximetry monitoring, personnel able to perform advanced cardiovascular life support (ACLS), external defibrillator immediately available outside zone 4, and personnel able to program CIED available as defined by institutional protocol.5
ALTERNATIVE PACING SITES
Anesthesia professionals might encounter CIEDs that are aimed to provide cardiac physiologic pacing (CPP). CPP is any form of pacing that restores or preserves ventricular synchrony. CPP is further divided into conduction system pacing such as His bundle pacing, left bundle branch pacing, or cardiac resynchronization therapy (CRT). CRT is achieved with biventricular (BiV) pacing using a coronary sinus branch or epicardial left ventricular lead. The goal of CPP is to reduce heart failure that may be seen in patients who require a significant amount of ventricular pacing. Patients who require substantial ventricular pacing may develop pacing-induced cardiomyopathy. Patients who have His bundle pacing or left bundle branch pacing should be managed similarly to patients with traditional dual chamber pacemakers in the perioperative period.
UPDATES IN THE LITERATURE
Ongoing Education to Manage CIEDs is Paramount
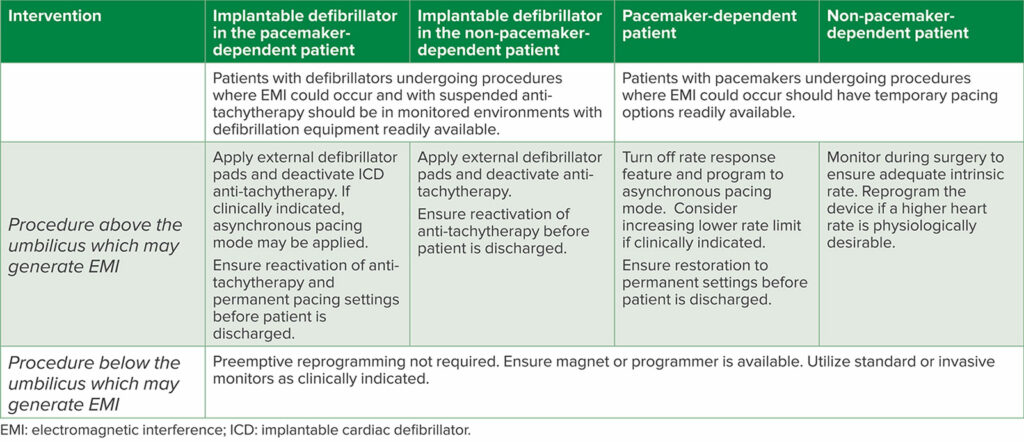
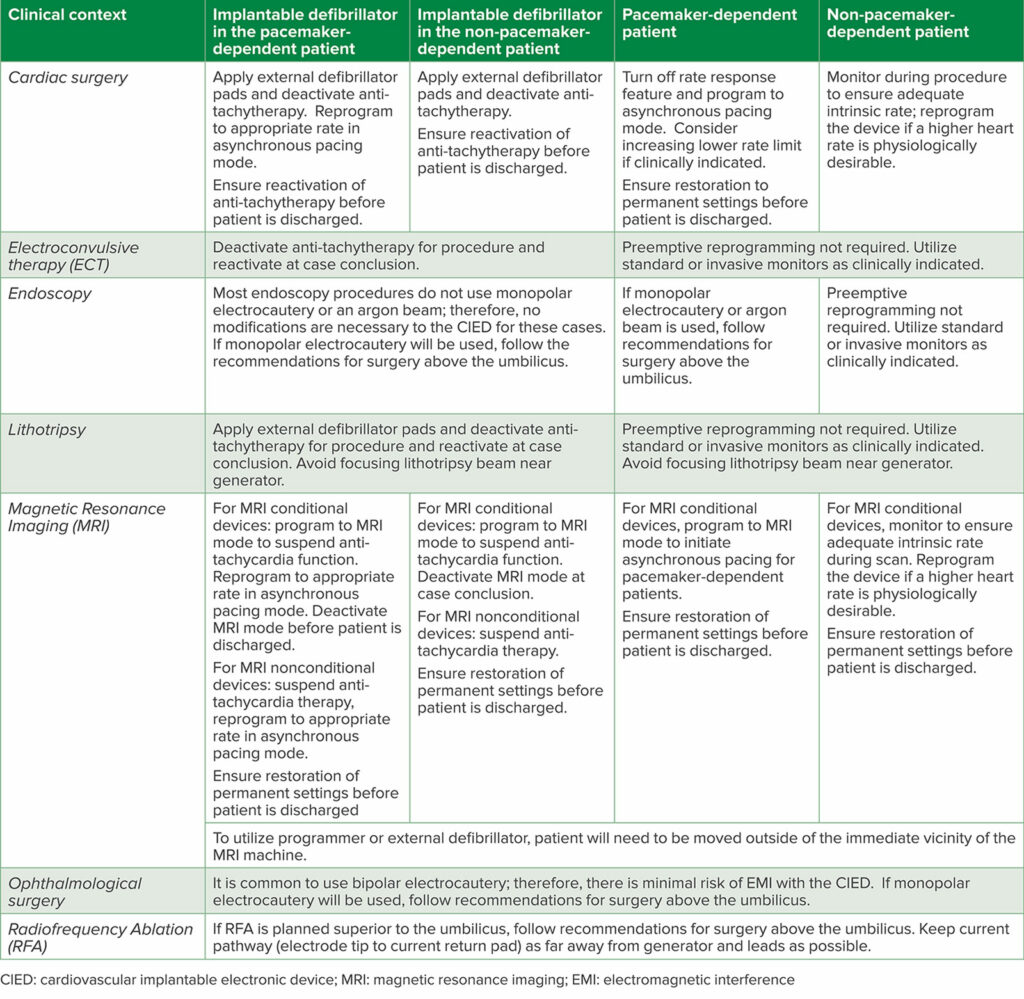
Since the 2020 update to the original article, further guidelines from the British Heart Rhythm Society have been published in 2022 in Anaesthesia.1,6,7 Additionally, the European Heart Rhythm Association in conjunction with the Heart Rhythm Society, Latin American Heart Rhythm Society, and Asian Pacific Heart Rhythm Society published a comprehensive consensus statement regarding the prevention and management of procedural EMI in patients with CIEDs (Table 2).8 Strengths of these articles include the discussion of common procedural contexts that have not before been broadly discussed, such as eye surgery, electroconvulsive therapy, and dental work, as well as more nuanced discussions of CIED management in clinical contexts like MRI scanning and therapeutic radiation for malignancy (Table 3).
Table 2: General Recommendations for Perioperative CIED Management
Table 3: Clinical Pearls for Perioperative CIED Management in Particular Contexts.
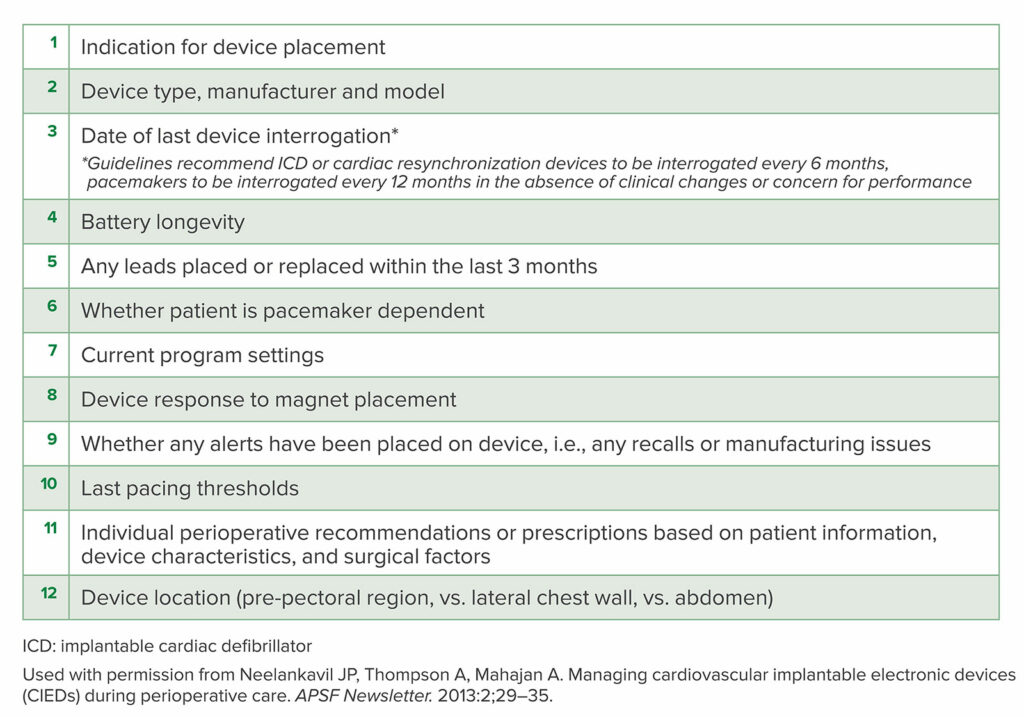
Key recommendations of these papers reiterate that electromagnetic interference, most often in the form of monopolar electrocautery above the umbilicus, may continue to pose a threat to patient safety by inhibiting pacing in the pacemaker-dependent patient, inappropriate cardiac defibrillator shocks, or device resets. The anesthesia professional at the time of procedure must be equipped with essential information (Table 4) to support the patient with a CIED through the periprocedural period. It is imperative the anesthesia team understand the response to a CIED to magnet application.
Table 4: Essential information to be communicated to the perioperative team by the CIED specialty or electrophysiology team
Although some academic centers have a dedicated perioperative CIED team,5 managing cardiac devices is within the scope of practice of perioperative providers.9 Thankfully, there exist guidelines and consensus statements to help steer perioperative management of these devices. Smartphone-based apps such as Pacemaker-ID and Device Detector may aid providers by being able to correctly identify CIEDs via chest x-ray. Especially as technology continues to evolve, ongoing education in the management of these devices is essential.
Andrew Disque, MD, is an associate professor in the Department of Anesthesiology and Perioperative Medicine at the David Geffen School of Medicine, UCLA.
Ashley P. Oliver, MD, MA, is an assistant professor in the Department of Anesthesiology and Perioperative Medicine at the David Geffen School of Medicine, UCLA.
Jacques P. Neelankavil, MD, is a professor in the Department of Anesthesiology and Perioperative Medicine at the David Geffen School of Medicine, UCLA.
The authors have no conflicts of interest.
REFERENCES
- Neelankavil JP, Thompson A, Mahajan A. Change of pace: an update on the perioperative management of cardiovascular implantable electronic devices (CIEDs). APSF Newsletter. 2020:35;92–93. https://www.apsf.org/article/change-of-pace-an-update-on-the-perioperative-management-of-cardiovascular-implantable-electronic-devices-cieds/ Accessed December 5, 2023.
- Bernstein AD, Daubert JC, Fletcher RD, et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing Clin Electrophysiol. 2002;25:260–264. PMID: 11916002.
- Practice advisory for the perioperative management of patients with cardiac implantable electronic devices: pacemakers and implantable cardioverter–defibrillators 2020: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Implantable Electronic Devices. Anesthesiology. 2020;132:225–252. PMID: 21245737.
- Indik J, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm. 2017;e97-e153. PMID: 28502708
- Streckenbach S, Lai Y, Bas H,, et al. Starting an anesthesia-based perioperative device management service: a practical guide to training anesthesiologists. J Cardiothor Vasc An. 2021;35:1006–1017. PMID: 33341343.
- Neelankavil JP, Thompson A, Mahajan A. Managing cardiovascular implantable electronic devices (CIEDs) during perioperative care. APSF Newsletter. 2013:28;29–35 https://www.apsf.org/article/managing-cardiovascular-implantable-electronic-devices-cieds-during-perioperative-care/ Accessed December 5, 2023.
- Thomas H, Plummer C, Wright IJ, et al. Guidelines for the peri-operative management of people with cardiac implantable electronic devices: guidelines from the British Heart Rhythm Society. Anaesthesia. 2022;77:808–817. PMID: 35429334
- Stühlinger, M, Burri H, Vernooy K, et al. EHRA consensus on prevention and management of interference due to medical procedures in patients with cardiac implantable electronic devices: For the European Heart Rhythm Association (EHRA), Heart Rhythm Society (HRS), Latin America Heart Rhythm Society (LAHRS), Asian Pacific Heart Rhythm Society (APHRS). Europace. 2022;24:1512–1537. PMID: 36228183
- Song P, Rooke GA. Fundamental electrophysiology principles related to perioperative management of cardiovascular implantable electronic devices. J Cardiothor Vasc An. 2023.Oct 6:S1053-0770(23)00800-5. PMID: 37940457


 Issue PDF
Issue PDF PDF
PDF