Related Articles:
February 2024
Keeping Pace: 2023 Update on the Perioperative Management of Cardiovascular Implantable Electronic Devices (CIEDs)
October 2020
Change of Pace: An Update on the Perioperative Management of Cardiovascular Implantable Electronic Devices (CIEDs)

Pacemaker
Cardiovascular implantable electronic device (CIED) is a term that encompasses pacemakers for bradyarrhythmia treatment, implantable cardioverter defibrillators (ICDs) for tachyarrhythmia management, and cardiac resynchronization therapy (CRT) devices for systolic dysfunction with conduction delays. Cardiac arrhythmias have an estimated prevalence of 14.4 million patients in the United States, and they account for approximately 40,700 deaths annually.1 As the indications for device placement continue to expand and with data supportive of device placement compared to medical therapy well established, CIEDs are becoming common in our patient population.2,3 Approximately one million patients worldwide receive a pacemaker or implantable cardioverter defibrillator (ICD) each year; therefore, it is imperative that all anesthesiolgists and anesthesia professionals understand the perioperative implications of these devices.
The 2011 Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement was a joint collaboration with the American Heart Association, the American College of Cardiology and the Society of Thoracic Surgeons, and it provides detailed information on a team approach to the management of CIEDs perioperatively. In this article, we review the contents of the consensus statement in addition to an overview of the management of CIEDs.
Perioperative Considerations
Preoperative Assessment
The HRS/ASA consensus statement concludes that most patients with CIEDs do not need a new preoperative evaluation by the CIED management team (physicians and other health care professionals who monitor the who monitor the CIED function of the patient) because, most of the time, the pertinent information will be available in the notes from the CIED clinic.4 Many patients with CIEDs have telephone interrogations every few months and yearly evaluations by their cardiologist. There are several things that an anesthesia professional should know about the CIED before taking the patient for surgery including what type of device the patient has, as that will guide the perioperative management.
Pacemakers are devices placed for bradyarrhythmias, and they remain the only effective treatment for ameliorating symptomatic bradycardia due to sinus node dysfunction (e.g., sick sinus syndrome) or a failure of impulse propagation (e.g., complete heart block). It is important to establish if the patient is pacemaker dependent, which is defined as the absence of a perfusing rhythm without pacing. If the patient is deemed to be pacemaker dependent, it is important to establish a secondary method for pacing the patient should a pacemaker failure occur. Alternative methods of pacing patients intraoperatively include transesophageal pacing, transcutaneous pacing, or transvenous pacing through a pacing pulmonary artery catheter or through a temporary transvenous pacing wire. Whatever method is chosen, it is important to have the necessary equipment and support organized and/or available prior to beginning the procedure.
Pacemakers have many additional features that correspond to the changing needs of patients throughout the day including rate responsiveness to increase pacing during times of increased physical exertion and sleep functions to decrease pacing rate during times of rest. In general, these rate enhancements should be disabled preoperatively.
ICDs have 4 main functions. They sense atrial or ventricular electrical activity, classify these signals to various programmed “heart rate zones,” deliver tiered therapies to terminate ventricular tachycardia or fibrillation, and pace for bradycardia. The most important aspect of ICD management preoperatively is deactivating the tachycardia response of the device to avoid inappropriate pacing or shocks due to electromagnetic interference. It must be noted that while the ICD’s defibrillating capabilities are disabled, it is critical to have other means of defibrillation immediately available. Surface electrocardiogram and adhesive defibrillator pads allow for optimal monitoring and the ability to defibrillate should the need arise. Regarding the pacing capabilities of a device, the same management guidelines for pacemakers outlined above should be followed.
With biventricular ICDs (also referred to as cardiac resynchronization devices), ventricular pacing optimizes ejection fraction. Cardiac resynchronization therapy (CRT) has been shown to decrease myocardial oxygen consumption while improving stroke volume in patients with low EF, significant intraventricular conduction delay, or interventricular dyssynchrony.5 In this clinical scenario, continuing to pace provides better hemodynamic stability than simply turning off the device.
The general recommendations made regarding preoperative assessment of CIEDs provide structure for anesthesia professionals caring for these patients, but it is important to remember that the HRS/ASA consensus statement stresses individualized care of each patient through clear communication between the anesthesia professionals, surgeon, and CIED team. The consensus emphasizes that a single recommendation for all CIED patients is not appropriate. It is extremely important that the surgical or procedural team communicate with the CIED team to identify the type of procedure and likely risk of electromagnetic interference, and the CIED team should communicate with the procedure team to deliver a prescription for the perioperative management of patients with CIEDs.
Electromagnetic Interference
Electromagnetic interference (EMI) can cause malfunction of pacemakers and defibrillators.6-8 There are several potential causes of EMI perioperatively including TENS units and electroconvulsive therapy; however, the most common cause of EMI for patient with CIEDs is monopolar electrocautery. EMI can cause pacing inhibition, damage the pulse generator, and cause inappropriate tachycardia therapy depending on the type of CIED, especially if the EMI is in close proximity to the pulse generator (within 6 inches). Bipolar electrocautery is not a concern for CIEDs since the current is small and energy travels between the 2 poles of the pen or stylus.9 However, bipolar electrocautery is usually used in microsurgery (ophthalmology or neurosurgery), which represents a minority of surgical cases. Bipolar electrocautery is only capable of coagulation whereas monopolar cautery may be used for dissection and coagulation, which is why it is more commonly used.
Current CIEDs have sophisticated algorithms to minimize inappropriate sensing and pacing from EMI, and in addition lead and generator design has improved to the point where reports of inappropriate CIED function during EMI are less common. However, it is important to understand how EMI may affect the intraoperative performance of CIEDs.
EMI can be interpreted by a pacemaker as intrinsic cardiac activity; in this setting it will not trigger a paced rhythm even though the patient may need to be paced. This is called oversensing. Oversensing with an ICD secondary to EMI may lead to inappropriate antitachycardic therapy (pacing or defibrillation) if the ICD interprets the EMI as a tachyarrhythmia.10 Inappropriate defibrillation may trigger a ventricular arrhythmia or may result in patient movement if the patient is not paralyzed during the anesthetic. New CIED algorithms are better at filtering EMI, but misinterpretation does occur.
It is recommended that if monopolar cautery is used, it should be used in short bursts of several seconds. There are several reasons for this recommendation. The arrhythmia detection for ICDs usually requires several seconds of tachycardia detection before antitachycardic pacing or defibrillation is instituted. Pauses in monopolar cautery allow for fewer erroneous ICD interventions. In addition, patients who are pacemaker dependent are less likely to have hemodynamic instability if their pacemaker oversenses the EMI and does not pace the patient for several short bursts as opposed to a long continuous monopolar cautery application. The cautery dispersion pad should be placed on the patient in a way that the path of EMI does not cross over the CIED generator.
For surgery below the umbilicus, the HRS/ASA statement recommends that there is minimal need to reprogram a CIED or place a magnet on the CIED because the risk of oversensing, generator damage, or lead damage is small. Magnets may still be used, but it is vital to understand the different magnet responses for CIEDs.
Magnets
Magnets have been used in the perioperative period as a way to convert pacemakers into an asynchronous mode; however, the magnet response is extremely variable depending on the device, the manufacturer, and the individual settings determined by the CIED team. Historically, magnets were intended to help interrogate devices and determine battery life, but they are currently used most often to prevent inappropriate oversensing by pacemakers and ICDs.
Magnet response varies depending on whether the device is a pacemaker or ICD. For pacemakers, the magnet response can be programmed by the CIED team. Therefore, some pacemakers will have no response when a magnet is placed and some pacemakers will pace asynchronously. The rate at which the pacemaker paces when the magnet is placed depends on the manufacturer and the battery life of the generator. If the battery life is low, the pacemaker will pace at lower rates, which may not be adequate for the perioperative period. Patients with pacemakers coming for major surgery may need higher pacing rates than they typically require in their daily life. The lower rate limit for many patients with pacemakers is usually 60-70; however, a normal response to decreased systemic vascular resistance and hypovolemia is an increase in heart rate. Although placing a magnet may place the patient into an asynchronous mode, the rate may not meet the physiologic demands of the patient.
For ICDs, magnet application will prevent both antitachycardic pacing and defibrillation in order to prevent oversensing of EMI, which may result in inappropriate tachycardia therapy. It is important to remember that all modern ICDs are also pacemakers; however, there is a critical difference in function when a magnet is applied to an ICD versus a pacemaker. In general, a magnet applied to an ICD generator will disable tachycardia therapy; however, it will not have any effect on the pacemaker. Therefore, magnet application to an ICD will NOT place the underlying pacemaker in an asynchronous mode (AOO, VOO, or DOO). For patients who are pacemaker dependent and have ICDs who are undergoing surgery where there is potential for significant EMI, it is best to reprogram the CIED to address both the tachycardic and bradycardic therapy.
A magnet’s effect on a CIED can be programmable in some devices, meaning that some devices will not display a typical magnet behavior when a magnet is applied to the device. Due to this varied magnet response depending on the type of CIED, manufacturer, and individual electrophysiologist inserting the device, it is important to confirm the magnet effect on each individual patient’s device prior to any operative procedure whenever possible.
CIED Failure
CIED failure is a rare perioperative occurrence that can result from a failure of the device to sense, a failure to pace, or damage to the generator. Most perioperative events that are thought to be pacemaker failures are really rate adaptive features that have not been disabled. For example, current pacemakers have minute ventilation sensors that increase the pacing rate for patients during exercise. EMI can change body impedance which might cause the pacemaker to pace at a fast rate since the pacemaker “sees” the EMI as increased physiologic demand.11,12
Electrical reset is also a very rare occurrence that can happen when EMI directly contacts the CIED generator and results in device failure. Therapeutic radiation is the usual perioperative culprit, and it is rare in the setting of monopolar cautery or cardioversion.13-15 If electrical reset does occur, each CIED, depending on manufacturer and device, will default to a particular setting. While the default setting may not be optimal for one’s specific patient, it will function safely until the device can be interrogated to determine if it can be reprogrammed or replaced. Damage to the generator may also be caused by electrocautery applied to the generator; therefore, the path of EMI should be directed away from the generator to prevent current flow across the device.
CIED leads may be damaged intraoperatively, leading to failures in sensing and/or pacing. EMI may produce enough current to flow from the generator to the pacing electrode and could possibly damage the tissue-lead interface. This acute injury may lead to loss of pacing and sensing.
Perioperative Management for Patients with CIEDs Presenting for Non-Urgent Surgery
Patients presenting for non-urgent surgery should have an algorithm of information that is communicated between the surgical, anesthesia, and CIED team (Table 1). Pacemakers should be interrogated every 12 months and ICDs and CRT devices should be evaluated every 6 months since ICD and CRT patients tend to have more significant co-morbidities. The CIED team should know the type of procedure, the patient position, the type of EMI that will be used, anticipated cardioversion, and post-operative disposition in order to make recommendations. Anesthesia professionals should know what type of device the patient has (pacemaker vs. ICD), the indication for placement, battery life documented greater than 3 months, the programming mode (i.e., DDD, DOO), pacemaker dependence and underlying rhythm, and the magnet response.
Understanding these variables will help the anesthesia provider understand the CIED team recommendations regarding the use of a magnet versus pacemaker reprogramming. In general, procedures below the umbilicus do not require CIED reprogramming, although prophylactic magnet application may be used if the magnet response is known to the anesthesiologist (Figure 1). For patients having surgery above the umbilicus, it is important to disable ICD tachycardia therapy and for patients with pacemakers, rate responsiveness should be disabled. For patients who are pacemaker dependent having surgery above the umbilicus, they should be reprogrammed to an asynchronous mode either via the CIED team or by magnet placement if patient positioning and surgical access allows. For patients with CRT, asynchronous pacing should be guaranteed for surgeries above the umbilicus since biventricular pacing for this subset improves cardiac output. For procedures below the umbilicus, patients with CRT do not need reprogramming.
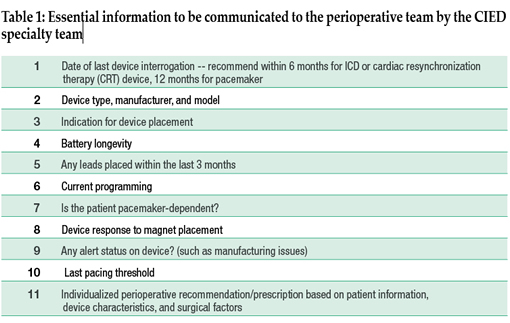
Emergency Management
For patients presenting for urgent or emergent surgery, there may not be sufficient time for the CIED team to make recommendations depending on the type of practice environment. In this setting, the anesthesia provider(s) should identify the type of device (pacemaker vs. ICD vs. CRT). There are several ways to obtain this information including medical records and patient CIED information card. If neither of these options is available, a chest radiograph can provide a great deal of information (see figure). Pacemakers have leads with consistent texture and thickness on radiographs but ICDs have shocking coils toward the distal tip of the lead which are brighter on radiograph and are thicker. Patients with CRT will have an additional lead that is entering the coronary sinus visible on the radiograph.
For patients having surgery below the umbilicus, one can proceed to surgery with the CIED device. For patients having surgery above the umbilicus, a preoperative 12-lead electrocardiogram or rhythm strip can determine if the patient is being paced. If pacing spikes are noted in front of most beats, one can assume the patient is pacemaker dependent. If there are no pacing spikes, one can proceed to surgery with a magnet in the room in case inappropriate sensing occurs. Monopolar electrocautery should be used in short bursts.

For ICDs, magnets should be used if the procedure is above the umbilicus to disable tachyarrhythmia therapy, but this will not change the pacemaker function. In this case, monopolar electrocautery should be used in short bursts to prevent pacemaker oversensing and resultant bradycardia in pacemaker dependent patients.
For emergencies, the CIED team should be contacted immediately. Even if there is not enough time to interrogate the device preoperatively, they can make intraoperative recommendations, and interrogate the device postoperatively. The 24-hour toll free phone contact number for all major CIED manufacturers should be readily available in the perioperative areas to all anesthesia providers (Table 2).
Conclusion
Anesthesiologists, as true perioperative physicians and other anesthesia professionals need to take an active role in learning about and managing these devices. It is important that all anesthesia providers understand the nuances to perioperative management of CIEDs, given that it is becoming increasingly difficult to obtain the consultative services of trained CIED specialists (cardiologists-electrophysiologists, manufacturer’s representatives, CIED therapy trained cardiac anesthesiologists), especially during emergencies and late hours/weekends. Prior understanding and knowledge of basic functioning of CIEDs along with their perioperative management will enable the anesthesia providers to better respond to patient care needs, as well as develop partnerships with the cardiology CIED teams in their institutions. Education in this area for all the anesthesia providers is an essential, but a challenging task. This needs to be accomplished through multiple sources such as local anesthesia training programs, web-based modules, simulation-based training, CIED workshop training by institutions and national societies, and national educational initiatives of multispecialty guideline development.
Clinical Vignette #1:
A 56-year-old male is admitted after a motor vehicle accident. He was intubated in the field, and is coming directly to the operating room for free air in the abdomen. A chest x-ray taken in the emergency department shows the following:
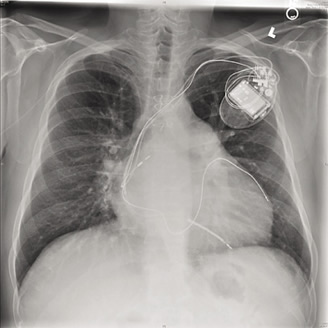
Teaching points: A chest x-ray can be extremely informative for patients coming for emergency surgery. A chest x-ray can identify the device type, leads, and manufacturer. From this x-ray, it is clear that the patient has 3 leads: a right atrial lead, a right ventricular lead, and a coronary sinus lead. In addition, the right ventricular lead is a shocking coil, which is identified by the thicker, denser distal portion of the lead. From this chest x-ray, it is clear that the patient has an ICD due to the shocking coil, and the coronary sinus lead suggests resychronization therapy for low ejection fraction. From this x-ray, this patient should be treated like any patient with cardiomyopathy. In addition, the emergency algorithm outlined above should be used to address the perioperative management of this ICD.
Clinical Vignette #2:
A 72-year-old female was admitted for acute abdominal pain. Surgical consultation and imaging led to a diagnosis of a small bowel perforation. She was urgently scheduled for surgery. During the history and physical, she commented that she had a pacemaker placed 1 month ago. She could not remember the details of why it was placed. A chest x-ray demonstrated the following:
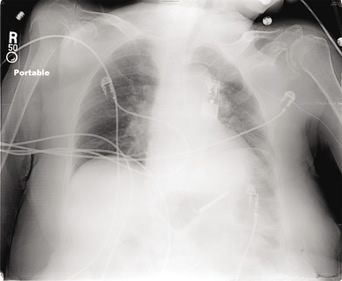
Teaching points: This device is actually a loop recorder placed to monitor heart arrhythmias for longer periods of time. As opposed to the x-ray above, there are no leads entering the heart. This patient does not need special management of this device in the perioperative period.

Jacques P. Neelankavil, MD is an Assistant Clinical Professor in the Department of Anesthesiology at the David Geffen School of Medicine at UCLA, Los Angeles, California.
Annemarie Thompson, MD, is an Associate Professor of Anesthesiology and Medicine and Co-Director of the Vanderbilt Preoperative Evaluation Center at Vanderbilt University School of Medicine, Nashville, Tennessee.
Aman Mahajan, MD, PhD, FAHA, is Professor of Anesthesiology and Bioengineering and the Ronald Katz Chair in the Department of Anesthesiology at the David Geffen School of Medicine at UCLA, Los Angeles, California.
References
- Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011;123:e18-e209.
- Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-83.
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L; Cardiac-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539-49.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. This document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS).
Heart Rhythm 2011;8:1114-54. - Ho JK, Mahajan A. Cardiac resynchronization therapy for treatment of heart failure. Anesth Analg 2010;111:1353-61.
- Belott PH, Sands S, Warren J. Resetting of DDD pacemakers due to EMI. Pacing Clin Electrophysiol 1984;7:169-72.
- Godin JF, Petitot JC. STIMAREC report. Pacemaker failures due to electrocautery and external electric shock. Pacing Clin Electrophysiol 1989;12:1011.
- Mangar D, Atlas GM, Kane PB. Electrocautery-induced pacemaker malfunction during surgery. Br J Anaesth 1991;38:616-18.
- Lee D, Sharp VJ, Konety BR. Use of bipolar power source for transurethral resection of bladder tumor in patient with implanted pacemaker. Urology 2005;66:194.
- Casavant D, Haffajee C, Stevens S, Pacetti P. Aborted implantable cardioverter defibrillator shock during facial electrosurgery. Pacing Clin Electrophysiol 1998;21:1325-6.
- Van Hemel NM, Hamerlijnck RP, Pronk KJ, et al. Upper limit ventricular stimulation in respiratory rate responsive pacing due to electrocautery. PACE 1989;12:1720-23.
- Wong DT, Middleton W. Electrocautery-induced tachycardia in rate-adaptive pacemaker. Anesthesiology 2001;94:710-11.
- Furman S, Fisher JD. Endless loop tachycardia in an AV universal [DDD] pacemaker. Pacing Clin Electrophysiol 1982;5:486-9.
- Katzenberg CA, Marcus FI, Heusinkveld RS, Mammana RB. Pacemaker failure due to radiation therapy. Pacing Clin Electrophysiol 1982;5:156-9.
- Rozner M. Pacemaker misinformation in the perioperative period: programming around the problem [comment]. Anesth Analg 2004;99:1582-4.


 Issue PDF
Issue PDF