Perioperative complications are more common in patients with SCD compared to the general population with an increased risk of postoperative acute chest syndrome (ACS), infections, vaso-occlusive pain crises, and 30-day mortality. Children with SCD have a different perioperative risk profile from adults because of the cumulative effect of sickled RBCs on end-organ dysfunction. Although the current evidence supporting routine preoperative transfusion in children with SCD is low-quality and based on low-to-moderate risk surgery, the preoperative transfusion plan should be patient-specific and consider the SCD genotype, baseline hemoglobin, disease severity, risk classification of the surgery, and history of prior surgical complications.
There is an ongoing focus on delivery of safe and high-quality care to patients in anesthesiology. Patient optimization prior to receiving an anesthetic is crucial to ensuring optimal patient care. The optimization of the pediatric patient with sickle cell disease (SCD) has been an area of continued interest, given its incidence and the perioperative implications of the disease. Children with SCD have a different perioperative risk profile from adults because of the cumulative effect of sickled RBCs on end-organ dysfunction.
SCD is a common hematologic defect with a substitution of valine for glutamic acid on the beta chain of hemoglobin, occurring in about 1 out of 365 African American births. In the United States, approximately 70,000 to 100,000 persons have SCD, with 2.6% of individuals of Mediterranean, Asian, and African origin affected.1 Patients may be either homozygous (HbSS), heterozygous (HbSC), or have an associated thalassemia (Hb-S-beta0 or Hb-S-beta+). The most severe clinical manifestations occur in patients with HbSS and Hb-S-beta0. The red blood cells (RBC) in these patients, when deoxygenated, undergo polymerization leading to RBC deformity (i.e., sickling), subsequent hemolysis, and vaso-occlusion.2 This RBC damage, precipitated by hypoxemia, hypothermia, hypovolemia, infection, pain, stress, and surgery can inhibit blood flow and cause ischemic injury, producing the symptoms of a sickle cell crisis, such as a pain crisis, acute chest syndrome, chronic organ damage, and musculoskeletal complications.
Surgery and general anesthesia pose challenges in maintaining homeostasis to decrease the physiologic triggers that may precipitate a sickle cell crisis. Children with SCD are at an increased risk for the following postoperative complications, with the incidence of an acute chest syndrome (ACS) of 3.08%, stroke of 0.2%, and 30-day mortality of 0.2%.3 Intravenous hydration, thermoregulation, and adequate oxygenation are part of the perioperative management aimed at preventing sickle cell crises.4,5 As with many circumstances, the clinical judgement of the perioperative team is imperative in the determination of the risk versus benefit of a preoperative transfusion in a patient with SCD.
The most common pediatric procedures are low-to-moderate risk (e.g. pressure equalizing tube insertion, laparoscopic cholecystectomy, tonsillectomy/adenoidectomy, laparoscopic splenectomy, umbilical hernia repair, laparoscopic appendectomy, and myringotomy tubes) as compared to adults who may undergo more high-risk procedures (e.g., cardiac surgery and cerebral revascularization).4- 10 In addition, limiting unnecessary blood transfusions in children is a significant consideration to avoid alloimmunization, volume overload, and immunosuppression.11-13 The incidence of alloimmunization in SCD ranges from 7% to 58% depending on age, number of previous transfusions, and use of red cell phenotypic matching. Children with a history of multiple alloantibodies, delayed hemolytic transfusion reaction , and/or hemolysis have an increased risk of adverse outcomes secondary to transfusion; therefore, careful consideration should be given prior to any transfusion.14,15
Controversy Remains Over the Appropriate Preoperative Transfusion Strategy in SCD Patients
The decision to administer a preoperative blood transfusion is part of the optimization strategy for SCD patients by hematologists and anesthesia professionals to decrease the percentage of sickled RBCs. The hope is to potentially decrease the risk of perioperative complications, especially in high-risk SCD patients; however, the literature around this topic has shown ambiguous results.5 Even though the American Society of Hematology 2020 guidelines suggest a preoperative transfusion to a hemoglobin level of 9 or 10 g/dL in all patients with SCD undergoing operations requiring general anesthesia lasting more than one hour, there still remains controversy over the appropriate preoperative transfusion strategy given the current evidence.6
There are limited studies in children on preoperative transfusions in children with SCD. For example, the Transfusion Alternatives Preoperatively in Sickle Cell Disease trial was a randomized controlled trial comparing the incidence of perioperative complications in patients who did or did not receive a preoperative transfusion. The trial, which included both adults and children, reported a lower incidence of perioperative complications in patients who were transfused preoperatively versus those who were not transfused.7 The transfusion arm was either 1) a simple transfusion to increase the hemoglobin (Hgb) transfusion to 10 g/dL in those patients with a Hgb less than 9 g/dL or 2) a partial exchange transfusion to decrease the Hgb S (Sickle cell Hemoglobin) percentage to less than 60% in those patients with a Hgb greater than 9 g/dL. Those patients who received a preoperative transfusion had a lower risk of postoperative acute chest syndrome and life-threatening complications (p = 0.023). There was no difference in postoperative pain crisis, hospital length of stay, or readmission rates. This study was small (n = 67) and heterogeneous, 40 children and 27 adults, making it difficult to quantify the benefit of preoperative transfusion in children with SCD.
While the above study was aimed at simple versus partial exchange transfusions, another randomized multicenter trial has evaluated outcomes in SCD patients following simple versus exchange transfusion.5 Participants in this study were randomized preoperatively to receive either an exchange transfusion regimen to decrease the Hgb S level to less than 30%, or a regimen with a simple transfusion to increase the Hb level to 10 g/dL. Cholecystectomy, head and neck surgery, and orthopedic surgery were the most common procedures in the study with children comprising over 90% (n = 502) of the cohort. Transfusion-related complications occurred in 14% of the exchange transfusion arm and 7% in the simple transfusion arm. The incidence of postoperative acute chest syndrome was 10% in both groups.5 A simple transfusion was as effective as an exchange transfusion at preventing perioperative complications in patients with SCD.
The observations reported by the above studies, however, differed from a study evaluating outcomes data related to SCD and blood transfusions from the American College of Surgeons NSQIP (National Surgical Quality Improvement Program) Pediatric database. In that study, a retrospective cohort of 357 children with SCD, undergoing low to moderate risk surgery (laparoscopic cholecystectomy, splenectomy, or appendectomy), suggested no difference in 30-day readmission rates, surgical site infections, wound dehiscence, pneumonia, unplanned reintubation, venous thromboembolism, urinary tract infection, postoperative transfusion, cardiac arrest, stroke, sepsis, and death in children who were transfused preoperatively versus those who were not transfused (p = 0.80).8 The 30-day rate of surgical complications did not differ between the groups (p = 0.84). Further subgroup analysis, defined by either a preoperative hematocrit greater than 27.3% or less than 27.3%, showed no difference in postoperative sickle cell crisis in those children who were transfused versus those who were not transfused. Preoperative transfusion, additionally, was not associated with a reduced rate of postoperative transfusions in this cohort.
Routine Preoperative Blood Transfusion in Children with SCD Is Not Recommended
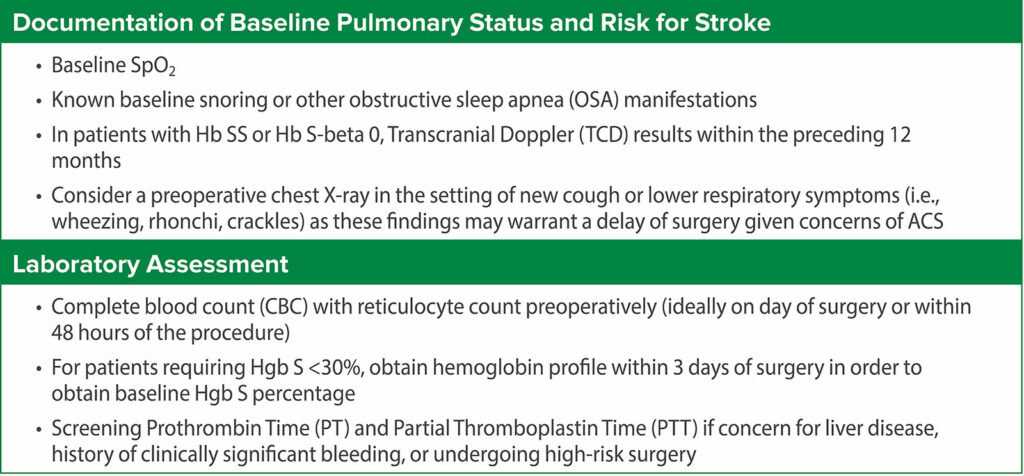
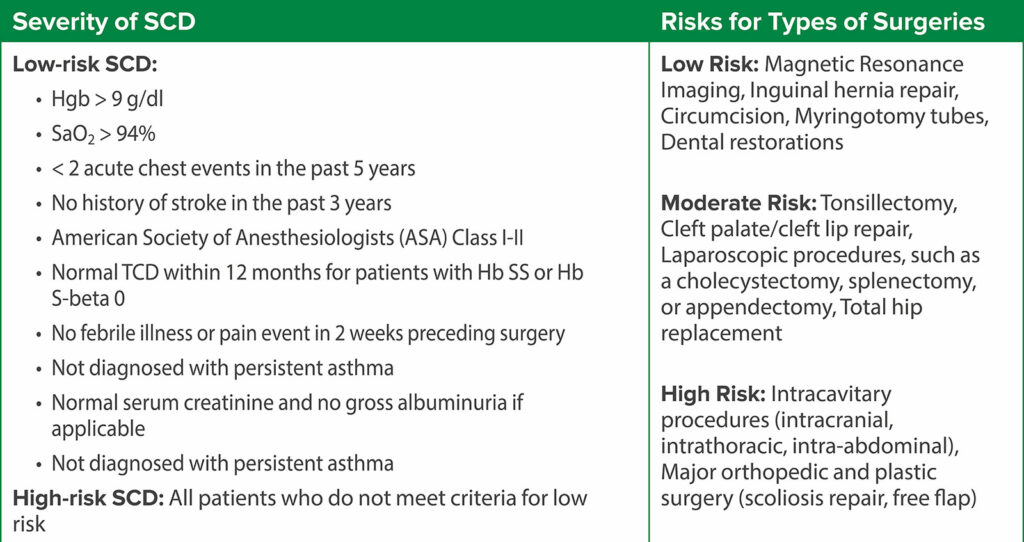
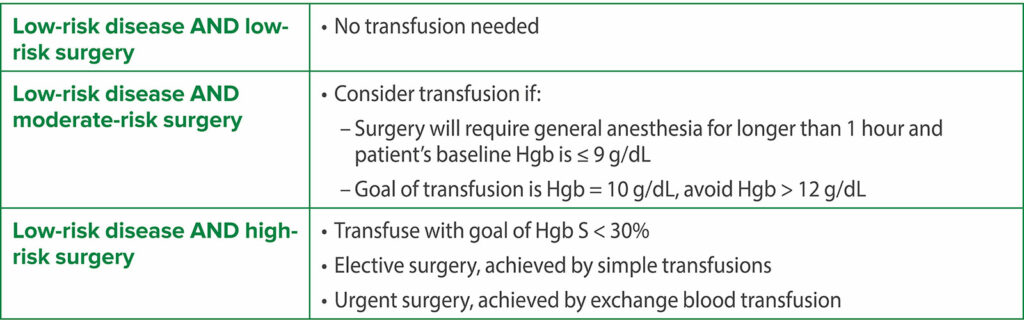
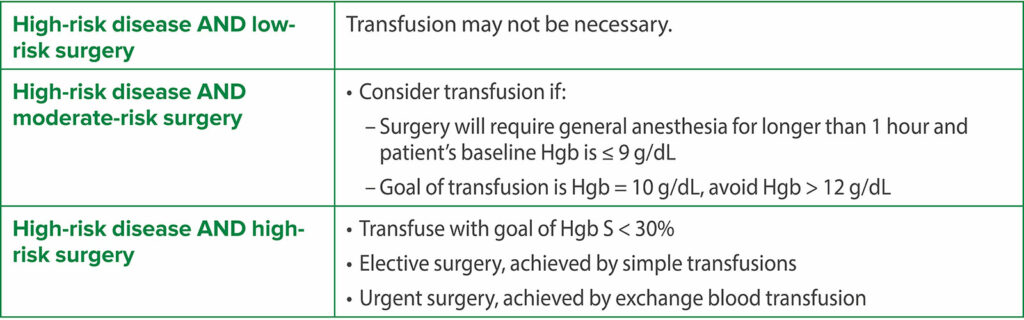
Thus, the current evidence supporting routine preoperative transfusion in children with SCD is inconsistent and inconclusive and does not favor routine preoperative blood transfusion. Hence, the decision for a preoperative transfusion should be patient-specific considering the SCD genotype, baseline hemoglobin, disease severity, risk classification of the surgery, and history of prior surgical complications. An interdisciplinary team, consisting of anesthesiology, hematology, and surgery, is important for perioperative management. An initial stepwise preoperative analysis should precede any decision to transfuse preoperatively (Table 1). The decision for transfusion depends on the risk categorization based on the severity of SCD and type of surgery (Table 2).4-6 A possible recommended plan based on these considerations is shown in Table 3 and Table 4 for low-risk SCD and high-risk SCD, respectively.7,9
Table 1: Preoperative Evaluation of the Child with SCD.16,17
Table 2: Risk Stratification Based on Disease Severity and Type of Surgery.16,17
Table 3: Plan for Low-risk SCD.16,17
Table 4: Plan for High-risk SCD16,17
In summary, the decision to transfuse children with SCD in the perioperative period should be guided by disease severity and the surgery category. Patients who may benefit from transfusion are patients at high risk for decompensation and include those who are either undergoing a high-risk procedure or at baseline have a high-risk disease state. Future research should focus on creating guidelines and protocols to guide clinicians as they strive to ensure safe and quality care in these high-risk patients.
Rahul Baijal, MD, is an associate professor in the Department of Pediatric Anesthesiology, Perioperative, and Pain Medicine, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Priti Dalal, MD, is a professor in the Department of Anesthesiology, Penn State Health & Penn State Children’s Hospital, Hershey, PA
Megha Kanjia, MD, is an associate professor in the Department of Pediatric Anesthesiology, Perioperative, and Pain Medicine, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
The authors report no conflicts of interest.
REFERENCES
- Khurmi N, Gorlin A, Misra L. Perioperative considerations for patients with sickle cell disease: a narrative review. Can J Anaesth. 2017; 64:860–869. PMID: 28455727.
- US Department of Health and Human Services. Evidence-based management of sickle cell disease: expert panel report, 2014. https://www.nhlbi.nih.gov/health-topics/evidence-based-management-sickle-cell-disease. Accessed March 2024.
- Hyder O, Yaster M, Bateman BT, Firth PG. Surgical procedures and outcomes among children with sickle cell disease. Anesth Analg. 2013;117:1192–1196. PMID: 24108258.
- Koshy M, Weiner SJ, Miller ST, et al. Surgery and anesthesia in sickle cell disease. Cooperative Study of Sickle Cell Diseases. Blood. 1995;86:3676–3684. PMID: 7579333.
- Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med. 1995;333:206–213. PMID: 7791837.
- Chou ST, Alsawas M, Fasano RM, et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv. 2020;4:327–355. PMID: 31985807.
- Howard J, Malfroy M, Llewelyn C, et al. The transfusion alternatives preoperatively in sickle cell disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet. 2013; 381:930–938. PMID: 23352054.
- Salvi PS, Solomon DG, Cowles RA. Preoperative transfusion and surgical outcomes for children with sickle cell disease. J Am Coll Surg. 2022;235:530–538. PMID: 35972175.
- Atwood CM, Gnagi SH, Teufel RJ, et al. Blood transfusion in children with sickle cell disease undergoing tonsillectomy. Int J Pediatr Otorhinolaryngol. 2017;103:117–120. PMID: 29224750.
- Oyedeji CI, Welsby IJ. Optimizing management of sickle cell disease in patients undergoing surgery. Hematology Am Soc Hematol Educ Program. 2021:405–410. PMID: 34889383.
- Chou ST, Jackson T, Vege S, et al. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013; 122:1062–1071. PMID: 23723452.
- Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood. 2007;21: 327–348. PMID: 17804128.
- Haberkern CM, Neumayr LD, Orringer, EP, et al. Cholecystectomy in sickle cell anemia patients: perioperative outcome of 364 cases from the National Preoperative Transfusion Study. Blood. 1997;89:1533–1542. PMID: 9057634.
- Chou ST, Jackson T, Vege S, et al. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–1071. PMID: 23723452.
- Lasalle-Williams M, Nuss R, Le T, et al. Extended red blood cell antigen matching for transfusions in sickle cell disease: a review of a 14-year experience from a single center (CME). Transfusion. 2011;51:1732–1739. PMID: 21332724.
- Schyrr F, Dolci M, Nydegger M. Perioperative care of children with sickle cell disease: a systematic review and clinical recommendations. Am J of Hematol. 2020;95:78–96. PMID: 31456233.
- Perioperative Management of Children with Sickle Cell Disease, Best Practice Standard, Texas Children’s Hospital, Evidence-Based Outcome Center. https://www.texaschildrens.org/patients-families/safety-and-outcomes/clinical-standards. Accessed March 2024.


 Issue PDF
Issue PDF PDF
PDF