Sevoflurane and desflurane were introduced into the U.S. market in the early 1990s, with each having some concerns about their safety. Sevoflurane had a fresh gas flow restriction due to concerns for the formation of compound A and an associated renal tubular cell necrosis found in a rat model.1 Desflurane was noted to be an airway irritant and associated with laryngospasm, sympathetic activation, tachycardia, and hypertension.2–6 We reviewed the Food and Drug Administration (FDA) adverse event reporting system to determine if these early concerns found validity after 25 years of clinical use of sevoflurane and desflurane.
Introduction
 In this report, we have explored the FDA Adverse Events Reporting System (FAERS) database for sevoflurane and desflurane seeking evidence, or lack thereof, for adverse events during clinical use of these two volatile anesthetics. The safety of modern-day volatile anesthetics is generally accepted, but concerns about their safety were debated at the time of their introduction into clinical use and thus merit further evaluation. Justifying our efforts to use FAERS database are the historical adverse events from the use of older volatile anesthetics and neuromuscular blocking drugs that were revealed after their introduction into clinical practice.7, 8 When new drugs are approved for broad use in the clinical settings with a diverse patient population and multiple co-morbid conditions, the time-tested process called pharmacovigilance can reveal new safety concerns.9 As an example, halothane-mediated hepatitis and enflurane-induced renal concentrating defects were first identified after these anesthetic gases were placed into the clinical setting.
In this report, we have explored the FDA Adverse Events Reporting System (FAERS) database for sevoflurane and desflurane seeking evidence, or lack thereof, for adverse events during clinical use of these two volatile anesthetics. The safety of modern-day volatile anesthetics is generally accepted, but concerns about their safety were debated at the time of their introduction into clinical use and thus merit further evaluation. Justifying our efforts to use FAERS database are the historical adverse events from the use of older volatile anesthetics and neuromuscular blocking drugs that were revealed after their introduction into clinical practice.7, 8 When new drugs are approved for broad use in the clinical settings with a diverse patient population and multiple co-morbid conditions, the time-tested process called pharmacovigilance can reveal new safety concerns.9 As an example, halothane-mediated hepatitis and enflurane-induced renal concentrating defects were first identified after these anesthetic gases were placed into the clinical setting.
As background, sevoflurane was released into clinical practice in the U.S. in 1995.10 The most important early safety concern with sevoflurane was the development of pentafluoroisopropenyl fluoromethyl ether (compound A), a breakdown product formed through the interaction of sevoflurane and carbon dioxide absorbents. Compound A’s effects had not been thoroughly investigated in patients in FDA Phase 1–3 trials.
This led to a 2 liter per minute (lpm) fresh gas flow (FGF) restriction to reduce human exposure to compound A. Studies in rat models indicated that Compound A could produce dose-dependent renal injury characterized by proximal tubular necrosis at inspired levels as low as 114 parts per million (ppm).1
Phase IV trials were subsequently performed in volunteers and patients undergoing long periods of sevoflurane anesthesia to examine both the level of exposure to Compound A, as well as to seek harmful effects based on clinical markers of renal function. In one such study, purposely designed to examine prolonged sevoflurane exposure at 1 lpm FGF, maximum compound A concentrations reached 34 ± 6 ppm, but no clinically significant changes in biochemical markers of renal dysfunction were found.11 Continued work revealed that humans were nearly devoid of an enzyme called renal beta lyase, a key enzyme directing the biodegradation of compound A to a toxic renal thiol in rats.12
Desflurane was introduced in 1992, and its low solubility in blood afforded it a clinical advantage of a more rapid induction and emergence from anesthesia and potentially a more rapid titration to a desired anesthetic depth compared with other volatile anesthetics in use. However, after the launch of desflurane, it was discovered that desflurane could activate airway reflexes because of its extreme pungency.2
Preclinical work with desflurane had also reported unexplained tachycardia and hypertension on occasion, and, in pediatric populations, bronchospasm. Desflurane’s lack of potency necessitated higher concentrations to achieve clinical effectiveness, thereby unmasking an adverse airway effect from its pungency. The adverse airway effect was associated with sympathetic activation during initial airway exposure after induction of anesthesia and during upward transitions in concenetration.2,3 Our lab had demonstrated a 2.5-fold increase in sympathetic nerve activity, hypertension, and tachycardia on initiation of desflurane after induction of anesthesia and an additional neurocirculatory activation with the transition from 1.0 to 1.5 MAC.4 Subsequent work indicated that nebulized lidocaine did not obtund the airway reflex response, but opioids had a dose dependent benefit in reducing the neurocirculatory activation.13,14
In this report, we have explored the FAERS database for sevoflurane and desflurane adverse events after a quarter century of clinical use in millions of patients.
We sought to determine if the self-reporting of adverse events to the FDA validated the initial concerns surrounding the safety of sevoflurane and desflurane and whether new safety concerns had been exposed during clinical use in a broad patient population.
Methods
In order to monitor drug safety in clinical practice, the Food and Drug Administration (FDA) has developed the FDA Adverse Event Reporting System (FAERS).15 FAERS is an online database the FDA uses to monitor all approved drugs and therapeutic biologic products by assessing the quantity, severity, and overall outcomes of new medications including volatile anesthetics. The FAERS database was queried for adverse events (AEs) reported for both sevoflurane and desflurane between 1996 and December 2019. Using demographic filters, AEs were analyzed for the two volatile anesthetics with each of the following age groups filters: 0 to 1-month-old, 2 months to 2-years-old, 3 to 11-years-old, 12 to 17-years-old, 18 to 64-years-old, 65 to 85-years-old, greater than 85-years-old, and age unspecified. AEs were sorted by Reaction Group. For example, the Reaction Group Cardiac Disorder, included specific reactions such as cardiac arrest, PEA, and ventricular tachycardia among others. For this article, general reactions Cardiac Disorders, Renal and Urinary Disorders, and Respiratory, Thoracic, and Mediastinal Disorders are reported in addition to specific reactions, e.g., ventricular tachyarrhythmias (ventricular tachycardia, ventricular fibrillation, torsades de pointes). Renal-specific disorders (oliguria, anuria, acute kidney injury/renal injury, renal impairment/failure, renal tubular disorder/dysfunction, and acute tubular necrosis) were summed to reduce the incidence of other less robust urinary reactions, e.g., urinary retention. All AEs are reported as a percentage of the total reaction group number for that anesthetic within each age group.
Results
Use of desflurane yielded 1140 reported AEs with the most common category (reaction group), being Injury, Poisoning, and Procedural Complications (24.9%). The top four subcategories within this reaction group included postprocedural complications, fetal exposure during pregnancy, anesthetic neurological complications, and awareness. Cardiac AEs were second at 23.9% with the most common subcategories including bradycardia, cardiac arrest, tachycardia, and ventricular tachycardia. Respiratory/Thoracic accounted for 19.4% with the top subcategory bronchospasm making up 2.9% of all desflurane-related AEs. The next most common were hypoxia, dyspnea, and laryngospasm.
Sevoflurane had 4977 reported AEs with the most common category being Injury, Poisoning and Procedural Complications (30.4%). The top four subcategories included anesthetic complications, postprocedural complications, anesthetic neurological complications, and awareness. As with desflurane, cardiac AEs were also the second most common reaction group for sevoflurane at 24.4%. The most common subcategories included cardiac arrest, bradycardia, tachycardia, and ventricular fibrillation. Respiratory/Thoracic events accounted for 18.7%, in similar range with desflurane. The four subcategories included pulmonary edema, hypoxia, apnea, and bronchospasm (1.6% of total sevoflurane AEs). Laryngospasm was the sixth most common subcategory in Respiratory/Thoracic complications. Of note, Renal-specific Disorders made up only 4.4% of all reported reactions for sevoflurane, compared with 5.3% for desflurane.
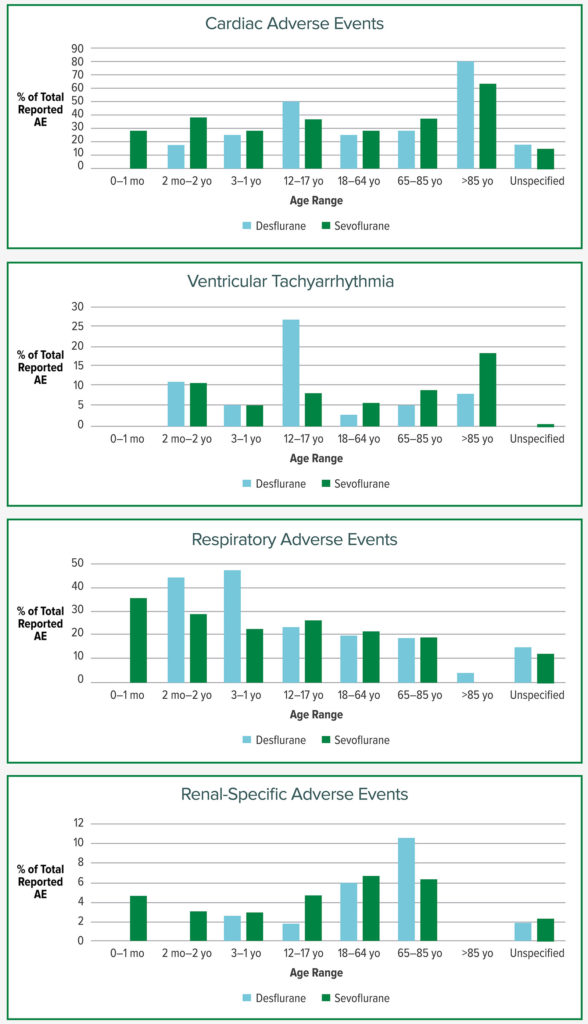
Figure 1 demonstrates AEs of interest for this report. Both desflurane and sevoflurane had high proportions of reported Cardiac AEs in the 85+ age group (80% and 63.6%). Ventricular tachyarrhythmia had a disproportionately high occurrence with desflurane in the age range of 12–17 at 26.8% compared to 8.2% for sevoflurane. There were very few reported adverse events in the neonatal age group for desflurane, although it tends not to be used in those under 2 years old due to distributor recommendations. The proportion of respiratory events out of total AEs for desflurane compared to sevoflurane were notably higher in the 2 months–2 years of age and 3–11 year age groups.
Discussion
The FAERS database contained 1140 adverse events reported for desflurane and 4977 reported for sevoflurane. The frequency of AE reporting is influenced by the total number of each of the anesthetics administered in clinical practice. But the AE reporting does confirm, by prevalence of AEs for each anesthetic, a number of areas of potential concern for these two volatile anesthetics in clinical use. Cardiac AEs were the second most commonly reported “reaction group” for both anesthetics. The proportion of ventricular tachyarrythmias was higher with desflurane in a younger population, but was noted to be higher with sevoflurane in an elderly population. Respiratory events were more prevalent than other adverse events in younger patients receiving desflurane. The greatest percentage of AEs in the Injury, Poisoning, and Procedural Complications group were awareness and neurologic AEs, likely capturing postoperative agitation and cognitive decline.
There are a number of links between the FAERS database and the clinical science for each anesthetic.
Arrythmias and Volatile Anesthetics: In vitro studies have shown that desflurane may increase intramyocardial catecholamine release,16 which could lead to the generation of arrhythmias. Desflurane has also been associated with more arrhythmias than sevoflurane after off-pump coronary artery bypass grafting,17 and has been associated with a higher rate of postoperative atrial fibrillation after on-pump cardiac surgery.18 The interlead variability of the QT interval, called the QT dispersion (QTd), is a marker for regional differences in ventricular repolarization and correlates better with the risk of dysrhythmia than the QT interval itself.19 In healthy adults undergoing noncardiac surgery, induction of anesthesia with only desflurane (no premedication) appeared to significantly increase the QTd while induction with only sevoflurane was not associated with any changes,20 but when midazolam and vecuronium were used prior to intubation, desflurane and sevoflurane both prolonged the QTd with no significant difference between the two.21 While a prolonged QTd can lead to various arrhythmias, its relationship to sympathetic activation, more commonly associated with desflurane is unknown.
Respiratory Disorders and Volatile Anesthetics: The proportion of respiratory AEs was high in the younger age group. As mentioned earlier, within the first few years of desflurane being available clinically there were concerns about pungency and airway irritation. As seen in recent studies regarding the respiratory effects of desflurane, a clear difference exists between adults and children. In an historical study of a large cohort of 14,000 children, researchers found that the use of desflurane was a risk factor for intraoperative respiratory events of all kinds, as well as for laryngospasm in particular.5 In a clinical trial of 400 healthy children who were randomized to either desflurane or isoflurane, children who received desflurane had a significantly higher frequency of airway events of any severity, laryngospasm, and coughing.6 However, the results are quite different when looking at adults. One meta-analysis of 13 randomized controlled trials showed no difference between sevoflurane and desflurane in the rates of upper airway events, laryngospasm, or cough at emergence.22 Another meta-analysis of seven randomized controlled trials showed no difference between sevoflurane and desflurane in the incidence of overall cough or laryngospasm in adults.23
Limitations
The FAERS relies on the voluntary reporting of adverse events by health care professionals and consumers in the United States, and for this reason there are important limitations to the database. First, there is no certainty that the reported adverse event was caused by the drug in question, as the FDA does not require that a causal relationship be proven. Second, the FDA does not receive every single adverse event that occurs for every single drug. There are many factors that determine whether a report will be filed, such as the severity or publicity of the event. It is expected that more serious side effects, such as cardiac arrhythmias, would be reported more frequently than other less serious reactions, such as postoperative nausea. For this reason, the database cannot be used to calculate an incidence of a given adverse event in the population. The frequency of use of sevoflurane is higher than desflurane in pediatric and adult patients.24 Thus, the total number of adverse events for any one volatile anesthetic is not relevant unless the denominator is precisely known.
Conclusions: Unlike other anesthesia drugs where their clinical use revealed new or unexpected safety concerns such as halothane hepatitis or anaphylaxis from rapacuronium, there do not appear to be any new or unexpected adverse phenomena after nearly 30 years of use of desflurane and sevoflurane. Early research identifying the neurocirculatory changes from the airway irritant effects of desflurane and the absence of renal injury from sevoflurane have carried forward to explain the findings in the FAERS self-reported data. Desflurane had a high incidence of airway events in a younger population that did not persist in older patients. Cardiac arrhythmias were noted with both anesthetics, and a prevalence of ventricular tachycardia was noted with desflurane in younger patients.
Thomas Ebert, MD, PhD, is a professor of anesthesiology at the Medical College of Wisconsin, Milwaukee, WI, and chief of anesthesiology at the Clement J. Zablocki Veterans Affairs Medical Center, Milwaukee WI.
Alex Ritchay, MD, is an anesthesiology resident at the Medical College of Wisconsin in the Department of Anesthesiology, Milwaukee, WI.
Aaron Sandock, BA, is a medical student at the Medical College of Wisconsin, Milwaukee, WI.
Shannon Dugan, BS, is a research assistant at the Medical College of Wisconsin, Milwaukee, WI.
The authors have no conflicts of interest.
References
- Gonsowski C, Laster M, Eger E, et al. Toxicity of compound A in rats. Anesthesiology. 1994;80:566–573.
- Muzi M, Lopatka C, Ebert T. Desflurane-mediated neurocirculatory activation in humans: effects of concentration and rate of change on responses. Anesthesiology. 1996;84: 1035–1042.
- Welskopf R, Moore M, Eger E, et al. Rapid increase in desflurane concentration is associated with greater transient cardiovascular stimulation than with rapid increase in isoflurane concentration in humans. Anesthesiology. 1994;80:1035–1045.
- Ebert T, Muzi M. Sympathetic hyperactivity during desflurane anesthesia in healthy volunteers. Anesthesiology. 1993;79:444–453.
- Oofuvong M, Geater A, Chongsuvivatwong V, et al. Risk over time and risk factors of intraoperative respiratory events: a historical cohort study of 14,153 children. BMC Anesthesiology. 2014;14:13.
- Lerman J, Hammer, G. Response to: Airway responses to desflurane during maintenance of anesthesia and recovery in children with laryngeal mask airways. Paediatr Anaesth. 2010;20:962–963.
- Habibollahi P, Mahboobi N, Esmaeili S, et al. Halothane-induced hepatitis: a forgotten issue in developing countries. Hepat Mon. 2011;11:3–6.
- Sosis MB. Further comments on the withdrawal of rapacuronium. Anesthesia & Analgesia. 2002;95:1126–1127.
- Sloane R, Osanlou O, Lewis D, et al. Social media and pharmacovigilance: a review of the opportunities and challenges. Br J Clin Pharmacol. 2015; 80:910–920.
- Smith I, Nathanson M, White P. Sevoflurane-a long-awaited volatile anaesthetic. Br J Anaesth. 1996;76:435–445.
- Ebert T, Frink E, Kharasch E. Absence of biochemical evidence for renal and hepatic dysfunction after 8 hours of 1.25 minimum alveolar concentration sevoflurane anesthesia in volunteers. Anesthesiology. 1998;88:601–610.
- Kharasch E, Jubert C. Compound A uptake and metabolism to mercapturic acids and 3,3,3-trifluoro-2-fluoromethoxypropanoic acid during low-flow sevoflurane anesthesia. Anesthesiology. 1999;91:1267–1278.
- Bunting H, Kelly M, Milligan K. Effect of nebulized lignocaine on airway irritation and haemodynamic changes during induction of anaesthesia with desflurane. Br J Anaesth. 1995;75:631–633.
- Pacentine G, Muzi M, Ebert T. Effects of fentanyl on sympathetic activation associated with the administration of desflurane. Anesthesiology. 1995;82:823–831.
- U.S. Food and Drug Administration. 2020. Potential signals of serious risks by FAERS. [online] Available at: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm565425.htm Accessed March 9, 2020.
- Park WK, Kim MH, Ahn DS, et al. Myocardial depressant effects of desflurane: mechanical and electrophysiologic actions in vitro. Anesthesiology. 2007;106:956–966.
- Hemmerling T, Minardi C, Zaouter C, et al. Sevoflurane causes less arrhythmias than desflurane after off-pump coronary artery bypass grafting: a pilot study. Ann Card Anaesth. 2010;13:116.
- Cromheecke S, ten Broecke PW, Hendrickx E, et al. Incidence of atrial fibrillation early after cardiac surgery: can choice of the anesthetic regimen influence the incidence? Acta Anaesthesiol Belg. 2005;56:147–54.
- Day C, McComb J, Campbell R. QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Heart. 1990;63:342–344.
- Yildirim H, Adanir T, Atay A, et al. The effects of sevoflurane, isoflurane and desflurane on QT interval of the ECG. Eur J Anaesthesiol. 2004; 21: 566-570.
- Kazanci D, Unver S, Karadeniz U, et al. A comparison of the effects of desflurane, sevoflurane and propofol on QT, QTc, and P dispersion on ECG. Ann Card Anaesth. 2009;12:107.
- Stevanovic A, Rossaint R, Fritz H, et al. Airway reactions and emergence times in general laryngeal mask airway anaesthesia. Eur J Anaesthesiol. 2015;32:106–116.
- de Oliveira G, Girao W, Fitzgerald P, et al. The effect of sevoflurane versus desflurane on the incidence of upper respiratory morbidity in patients undergoing general anesthesia with a Laryngeal Mask Airway: a meta-analysis of randomized controlled trials. J Clin Anesth. 2013;25:452–458.
- Nasr G, Davis M. Anesthetic use in newborn infants: the urgent need for rigorous evaluation. Pediatr Res. 2015;78: 2–6.


 Issue PDF
Issue PDF