Practices to Ensure Adequate Venous Access and Safe Drug Administration During Transfer to the Operating Room for Emergency Cesarean Delivery
On behalf of the Society for Obstetric Anesthesia and
Perinatology (SOAP) Patient Safety Committee
Patient transfer to the operating room for emergency cesarean delivery is a high risk epoch for serious medication error or venous access complication, based on a series of patient safety incidents reported to the Society for Obstetric Anesthesia and Perinatology (SOAP) Patient Safety Committee. Emergency cesarean delivery can be among the most time sensitive surgical procedures performed. In the rush to the operating room, well-intentioned intraprofessional teams have the potential to make any number of skill-based errors or mistakes with serious consequences. Even minor errors, such as venous access dislodgment, can produce life-threatening delays in this context.
Magnesium sulfate and oxytocin are included in the list of high risk medications by the Institute for Safe Medicine Practices (ISMP) (Table 1). Inadvertent bolus administration at the time of transfer to the operating room may be caused by pump programming errors or confusion between magnesium sulfate, oxytocin, and intravenous (IV) fluids used for hydration.1 Strategies for safe use of both magnesium and oxytocin are actively investigated. Medication administration on an infusion pump through dedicated, color-coded tubing without stopcocks or side-injection ports can reduce the risk of drug error. This facilitates a process by which the color coded tubing may then be rapidly disconnected from the main intravenous line, and capped, prior to emergent transfer to the operating room (Table 2).
Table 1: Institute for Safe Medication Practices (ISMP) High-Alert Medications† that are frequently administered on labor and delivery units.
| Epinephrine |
| Epidural or intrathecal medications |
| Insulin |
| Magnesium sulfate |
| Oxytocin |
| Nitroprusside |
| Potassium chloride |
| Promethazine |
† Medications that carry increased risk of patient harm when given improperly or in error. Table adopted from www. ismp.org and patientsafetyauthority.org.
Table 2. Emergency Cesarean Transport Procedure‡
| Disconnect all medication infusions, leaving crystalloid only attached to the main IV tubing |
| Cap all IV medication lines, the epidural infusion line, IV tubing stopcocks and side ports, and the epidural catheter; maintain appropriate caps at bedside (e.g., hanging from the IV pole) |
| Remove the main IV line from the infusion pump, and administer crystalloid by gravity to verify a free-flowing infusion; for inadequate flow, secure appropriate venous access either immediately or upon OR entry |
| During transport, position clamped IV line next to patient as close as possible to the IV insertion site to prevent inadvertent dislodgement during transport |
| Transport all IV and epidural infusion pump(s), medication solutions, and capped infusion lines to the OR separated from patient (e.g., hanging on an IV pole). |
IV = intravenous; OR = operating room; L&D = labor and delivery unit
‡Suggest training all L&D nurses to apply this procedure for any emergent transfer to the operating room.
Venous Access
Although many delivery units require minimum gauge venous access (e.g., 18 gauge),2 (Table 3), some patients refuse intravenous (IV) cannulation or present access difficulty resulting in smaller gauge IV placement. Patients should be counseled regarding the role of IV access during emergency care and the potential for worsened outcome should delivery or resuscitation be delayed due to lack of sufficient venous access. In patients who do not require or refuse IV fluids during labor, a heplocked IV can be placed to provide emergency access if needed. This can be covered with a water-tight occlusive dressing (e.g., Tegaderm) for parturients who wish to utilize a shower or birthing tub during labor.
Increasingly, units rely on infusion pumps to administer all fluids and medications during labor. Infusion pumps have the potential to mask IV infiltration, which is only recognized at the time of transfer to the operating room. To verify patency, teams should be trained to critically examine the insertion site at regular intervals, and to remove the carrier solution from the infusion pump to verify successful administration by gravity prior to transfer to the operating room (Table 2). To facilitate more rapid transfer to the operating room, and to limit the risk of dislodgement, the main carrier line can either be disconnected and capped, or clamped off, and positioned alongside the patient in the bed immediately adjacent to the intravenous insertion site.
Table 3: Potential hazards associated with medication and infusion management on labor and delivery, and suggested strategies to mitigate each hazard. (click table to get an enlarged page)
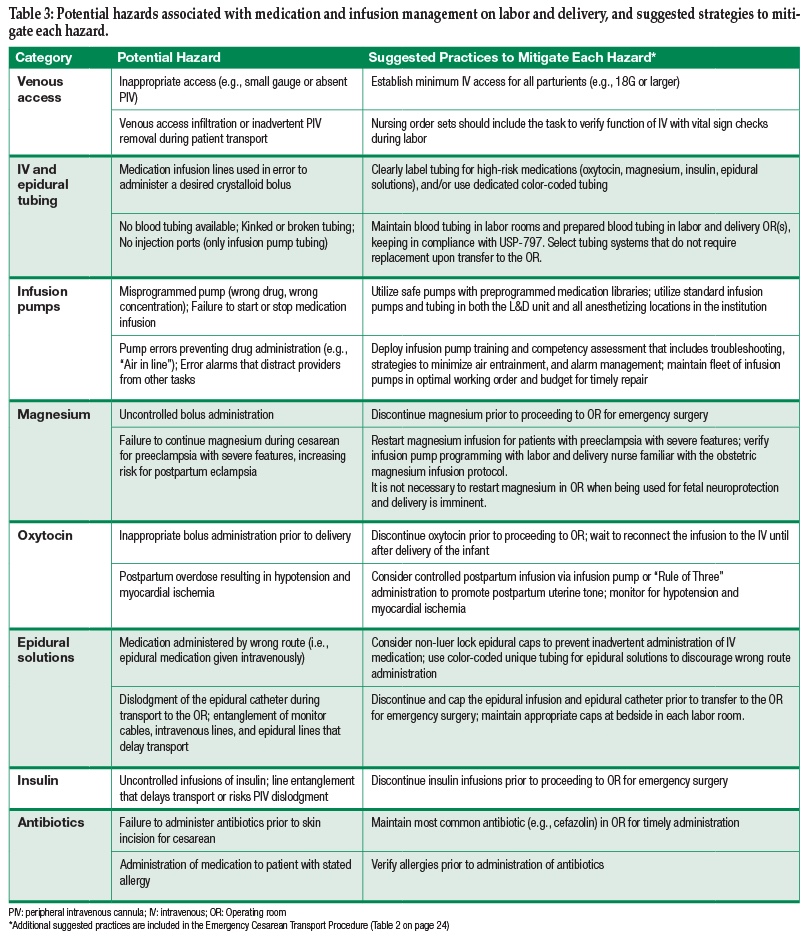
With some infusion pump systems, the intravenous tubing does not contain injection ports, or does not allow flow when disconnected from the infusion pump. As noted, these are desirable characteristics for the tubing used to administer high risk medications, but mandate tubing replacement prior to OR transfer for the main carrier line. Systems to facilitate rapid tubing replacement include assigning the responsibility to the anesthesia providers, advanced preparation of a tubing setup in the operating room keeping in compliance with United States Pharmacopeia chapter 797 for Pharmaceutical Compounding—Sterile Preparations,3 and widespread distribution of the equipment to prepare additional tubing setups. As health systems look increasingly towards efficiency and cost containment, tubing systems that function across clinical contexts will become increasingly important, and anestheisa professionals who engage with hospital equipment purchasing committees may be successful in identifying and selecting products that obviate the need to switch tubing altogether. To maximize equipment familiarity, the same type of IV tubing and infusion pumps should be utilized in all anesthetizing locations within an institution.
Infusion pumps are heavy and once multiple infusions are started, pose the risk of tangled lines and the need for additional equipment to hold the pump(s). Depending on the medication and indication, infusions often need to accompany the patient to the operating room. Epidural and intravenous infusion pumps loaded with medication solutions and capped tubing can be hung on a single IV pole, and transported to the operating room by any provider who is not simultaneously maneuvering the patient and bed (Table 2 and Table 3). This process helps prevent lines from further tangling, lines from being run over by the patient’s bed, and potential loss of an IV if the pole and infusion lines get caught on a door frame. Having infusions disconnected during transit for an emergency case also helps protect from accidental changes in drug administration by rushed care team members. For elective or urgent cesarean deliveries when there is additional time to prepare, good communication is needed between the anesthesia, obstetric, and nursing teams regarding whether or not to disconnect infusions during transport.
Management of Magnesium Infusions
Magnesium sulfate is indicated to reduce the risk of eclampsia in women with preeclampsia with severe features4 and also for neuroprotection for fetuses at less than 32 weeks estimated gestational age.5,6 According to one commonly recommended protocol for preeclampsia, a bolus of magnesium sulfate (4–6 grams) is followed by a continuous infusion (1–2 gram/hr),7 and the patient’s deep tendon reflexes are monitored for signs of early magnesium toxicity. Magnesium is considered a high risk medication due to the potential for sedation, respiratory depression, arrhythmia, and even cardiac arrest if given in excess.8-10 Accidental overdose of magnesium resulting in cardiac arrest has been reported during transport of a parturient to the operating room for emergency cesarean delivery.9 Magnesium sulfate also prolongs the duration of nondepolarizing neuromuscular blockade and bolus administration is a risk factor for uterine atony. Current American College of Obstetricians and Gynecologists (ACOG) recommendations support continuing magnesium infusion during delivery, whether vaginal or cesarean, in patients with preeclampsia with severe features.4 The half-life of magnesium is 5 hours; holding the infusion throughout a cesarean delivery may increase risk for postpartum maternal seizure.11 Thus, while the magnesium infusion should be discontinued during transport to the operating room for cesarean delivery, the infusion should be restarted as soon as feasible before or during the surgery (Table 3). Consultation with the labor and delivery nurse may be necessary to ensure appropriate infusion pump re-programming at the time of restarting the magnesium infusion.
When magnesium is given for fetal neuroprotection, depending on the regimen, the infusion is continued 12–24 hours or until birth, whichever occurs first.12,13 When a woman requires emergent cesarean delivery while receiving magnesium for fetal indications, there is no demonstrated benefit to maintaining the infusion to the moment of birth.13,14 As previously mentioned, the infusion should be discontinued for transport. Due to the long half-life of magnesium, serum levels will be maintained in both mother and fetus in the short time required for emergent cesarean delivery of the infant.
Management of Insulin Administration
Likewise, intrapartum insulin is almost always administered for fetal indications, specifically to prevent neonatal hypoglycemia. Given the imminence of delivery with emergent cesarean, insulin should be discontinued prior to transport to the operating room, without concern for significant impact on neonatal hypoglycemia (Table 3). Maternal blood glucose management can be addressed using protocols appropriate for all postoperative adult patients.
Management of Oxytocin Administration
Oxytocin administration is particularly hazardous because appropriate antepartum doses utilized for induction and augmentation of labor are an order of magnitude less than postpartum doses needed to promote uterine tetany and prevent postpartum hemorrhage. If large doses are given prior to delivery, uterine tachysystole can occur, impeding blood flow and oxygen delivery to the placenta and fetus, and necessitating urgent or emergency cesarean delivery.15 Because oxytocin is often infused from a 500 or 1000 mL bag, perinatal providers may mistake the oxytocin for a bag of crystalloid fluid and administer a fluid bolus in response to fetal bradycardia or maternal hypotension.16 Ideally, commercially or pharmacy-prepared bags of oxytocin solution are supplied in IV bags with a distinct size and shape not used for any other purpose on the unit. When providers prepare IV bags of oxytocin in bags of crystalloid commonly used on the unit (e.g., 30 Units/500-1000 mL), care should be taken to circumferentially label the bag to reduce the risk of confusion with plain crystalloid solutions. Similar to magnesium, oxytocin infusions should be disconnected from the patient prior to transport to the operating room for cesarean delivery (Table 3). The most conservative way to prevent premature administration is to wait to reconnect the oxytocin infusion to the intravenous line until after delivery of the infant.
Postpartum oxytocin is most commonly administered as a continuous infusion. Bolus administration and infusion rates above 1 unit/minute can result in hypotension, tachycardia, chest pain, and myocardial ischemia (e.g., ST changes on EKG, increases in troponin).17,18 The common practice of injecting concentrated oxytocin into a standard IV crystalloid bag (30 units in 500 or 1000 mL), and running the solution “wide-open” results in uncontrolled infusions that may easily exceed 1 unit of oxytocin per minute. The optimal post-cesarean oxytocin infusion protocol has yet to be determined, but infusions of 300 milliunits per minute maintained for 1 hour19 appear adequate to prevent clinically significant hemorrhage.20,21 Although small bolus doses of oxytocin (3 units administered every 3 minutes up to a total of 3 doses) appear to require less total drug to establish acceptable uterine tone when compared with oxytocin infusions,22 hypotension with myocardial ischemia may increase with this approach.18
For women undergoing unplanned cesarean delivery with risk factors for uterine atony, a brief period (1–5 minutes) of rapid controlled infusion (500 to 1000 milliunits per minute) could be used to establish uterine tone,17,23 followed by a reduced and more hemodynamically stable maintenance infusion. Following an initial dose of 5 units of oxytocin, the addition of a maintenance infusion (40 units over 4 hours) has been shown to reduce the requirement for secondary uterotonics, but did not have any measurable impact on the incidence of major postpartum hemorrhage.24
Team Training to Promote Medication Safety
Evidence supports the use of simulation to improve team performance during emergency situations on labor and delivery.25 Not only should clinical scenarios be practiced, but attention should also be paid to logistical issues regarding medication administration. During simulations of emergency cesarean, providers should practice preparing blood tubing, and disconnecting, capping, and reconnecting IV and epidural infusions as part of the process of moving the patient and infusion pumps to the operating room. Evaluation and maintenance of IV access should also be emphasized. Drills should be done with simulated patients on magnesium and/or oxytocin infusions so providers are familiar with safe handling of these high risk medications during times of high stress. Outside of simulations, teams can be trained to discuss medication issues either in a pre-operative huddle or during the operative time-out. Obstetricians and anesthesia providers should communicate which infusions will be stopped and which should be restarted and continued throughout the cesarean delivery.
While there are many potential hazards (Table 3) associated with medication administration on labor and delivery, the SOAP Patient Safety Committee has also identified several strategies and specific practices to mitigate these hazards. Vigilance, careful systems design, and a culture of robust communication between anesthesia, obstetric and nursing providers can help ensure safe care of parturients regardless of the medications they require during their time on labor and delivery.
The suggested practices presented in this article reflect the opinions and clinical experience of individual members of the SOAP Patient Safety Committee, and have not received official endorsement by SOAP or any another organization.
Contributors:
Christopher Ciliberto, MD; Eva Szabo, MD; Jennifer Banayan, MD; Gillian Hilton, MD; Steve Lipman, MD; Joanna Kountanis, MD
References
- Medication errors in labor and delivery: Reducing maternal and fetal harm. Pennsylvania Patient Safety Advisory 2009;6(Suppl 1):1-6. Available at: http://patientsafetyauthority.org/
ADVISORIES/AdvisoryLibrary/2009/dec16_6(suppl1)/Pages/01.aspx. Accessed August 11, 2015. - Kacmar RM, Mhyre JM, Scavone BM, et al. The use of postpartum hemorrhage protocols in United States academic obstetric anesthesia units. Anesth Analg 2014;119:906-10.
- American Society of Health-System Pharmacists. ASHP guidelines on compounding sterile preparations. Am J Health-Syst Pharm. 2014;71:145–66.
- Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. American College of Obstetricians and Gynecologists. 2013.
- Conde-Agudelo A, Romero R. Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks’ gestation: A systematic review and metaanalysis. Am J Obstet Gynecol 2009;200:595-609.
- Doyle LW, Crowther CA, Middleton P, et al. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev 2009;(1):CD004661.
- Lu JF, Nightingale CH. Magnesium sulfate in eclampsia and pre-eclampsia: pharmacokinetic principles. Clin Pharmacokinet 2000;38:305-314.
- Simpson KR, Knox GE. Obstetrical accidents involving intravenous magnesium sulfate: Recommendations to promote patient safety. MCN Am J Matern Child Nurs 2004;29:161-9.
- Morisaki H, Yamamoto S, Morita Y, et al. Hypermagnesemia-induced cardiopulmonary arrest before induction of anesthesia for emergency cesarean section. J Clin Anesth 2000;12:224-6.
- Bain ES, Middleton PF, Crowther CA. Maternal adverse effects of different antenatal magnesium sulphate regimens for improving maternal and infant outcomes: A systematic review. BMC Pregnancy Childbirth. 2013;13:195.
- Taber EB, Tan L, Chao CR, et al. Pharmacokinetics of ionized versus total magnesium in subjects with preterm labor and preeclampsia. Am Journal Obstet Gynecol 2002;186:1017-21.
- Crowther CA, Hiller JE, Doyle LW, et al. Effect of magnesium sulfate given for neuroprotection before preterm birth: A randomized controlled trial. JAMA 2003;290:2669-76.
- Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. New Engl J Med 2008;359:895-905.
- Reeves SA, Gibbs RS, Clark SL. Magnesium for fetal neuroprotection. Am J Obstet Gynecol 2011;204:202.e201-e204.
- Clark SL, Simpson KR, Knox GE, et al. Oxytocin: New perspectives on an old drug. Am J Obstet Gynecol 2009;200:35.e1-6.
- Simpson KR, Knox GE. Oxytocin as a high-alert medication: Implications for perinatal patient safety. MCN Am J Matern Child Nurs 2009;34:8-15.
- Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing Caesarean section. Br J Anaesth 2007;98:116-9.
- Bhattacharya S, Ghosh S, Ray D, et al. Oxytocin administration during cesarean delivery: Randomized controlled trial to compare intravenous bolus with intravenous infusion regimen. J Anaesthesiol Clin Pharmacol 2013;29:32-5.
- George RB, McKeen D, Chaplin AC, et al. Up-down determination of the ED(90) of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing Cesarean delivery. Can J Anaesth 2010;57:578-82.
- Dagraca J, Malladi V, Nunes K, et al. Outcomes after institution of a new oxytocin infusion protocol during the third stage of labor and immediate postpartum period. Int J Obstet Anesth 2013;22:194-9.
- Lee AI, Wong CA, Healy L, et al. Impact of a third stage of labor oxytocin protocol on cesarean delivery outcomes. Int J Obstet Anesth 2014;23:18-22.
- Kovacheva VP, Soens MA, Tsen LC. A randomized, double-blinded trial of a “Rule of Threes” algorithm versus continuous infusion of oxytocin during elective cesarean delivery. Anesthesiology 2015; In Press.
- Lavoie A, McCarthy RJ, Wong CA. The ED90 of prophylactic oxytocin infusion after delivery of the placenta during cesarean delivery in laboring compared with nonlaboring women: An up-down sequential allocation dose-response study. Anesth Analg 2015;121:159-64.
- Sheehan SR, Montgomery AA, Carey M, et al. Oxytocin bolus versus oxytocin bolus and infusion for control of blood loss at elective caesarean section: Double blind, placebo controlled, randomised trial. BMJ 2011;343:d4661.
- Lipman SS, Carvalho B, Cohen SE, et al. Response times for emergency cesarean delivery: Use of simulation drills to assess and improve obstetric team performance. J Perinatol 2013;33:259-63.


 Issue PDF
Issue PDF