As medical technology advances, so does the complexity of the environment for anesthesia care. Many specialty care centers are utilizing hybrid combinations of MRI, radiation, and lasers in operating suites and other patient care areas. Some of these new treatment and diagnostic modalities pose hazards to the patient, anesthesia provider, and the safe delivery of an anesthetic. In this segment, we will attempt to describe the challenges met at the MD Anderson Cancer Center during the design, implementation, and utilization of new cutting-edge technologies. Since many of these facilities are located a significant distance from the main operating room suite, they are considered remote locations. However, what makes them “extreme” locations is the fact that the anesthesia provider is usually separated from the patient during treatment and that team members must understand specific safety and logistical nuances to provide a safe anesthetic for the patient.
Intraoperative MRI (iMRI)
Intraoperative MRI or iMRI facilities are expanding from specialty care centers to regional hospitals as their costs become more economically feasible and their uses expand. As a National Cancer Institute (NCI) designated cancer center, we began operating in the iMRI in 2007. In the iMRI, a special table interfaces with a patient in neurosurgical pins, an MRI scanner, and a navigation system. Using this integrated system, surgeons are able to scan prior to surgical incision to load image data into the navigation system to guide the surgical approach. As the surgery progresses and resection ensues, subsequent scans are obtained intraoperatively to update the data to assess tumor remnants and also to create a reference for proximity to key structures.
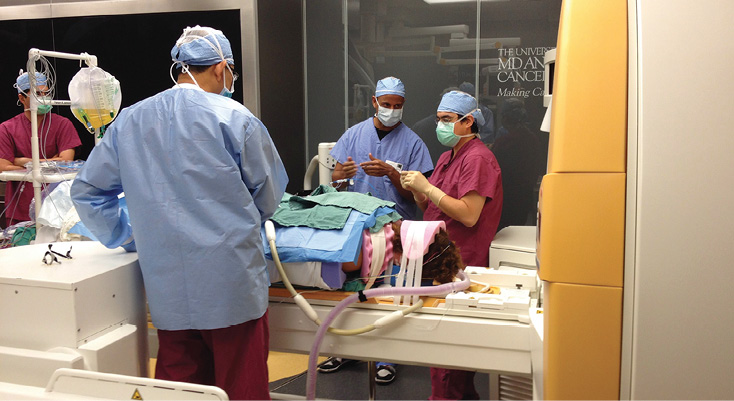
Laser Ablation with intraoperative MRI.
There are challenges to providing a safe environment for the patient and staff. Patient selection is important and is established through a questionnaire that focuses on possible ferrous containing implants such as pacemakers, metal joints, aneurysm clips, and even certain permanent make-ups and tattoos. Patient body habitus plays a factor as well, and an occasional “dry run” must occur to ensure the MRI scanner can accommodate the patient. Instrument counts are paramount before the surgery ensues and before every intraoperative scan including needle counts, so as to make certain that no instruments cross the 5 Gauss line (the outermost line that defines the limit beyond which ferromagnetic objects are strictly prohibited) and into the scanner. We utilize an induction room that is separated, by a door, from the MRI scanner environment so that during induction of anesthesia and invasive line placement, ferrous containing instruments like laryngoscope blades, handles and needles from line placement are well outside of the 5G area of protection. This induction room also serves as a designated area that houses resuscitative equipment and, in the event of an emergency, serves as an area in which cardiopulmonary resuscitation can take place safely. This room also allows for the assistance of additional personnel without exposure to the MRI environment.
Soon after establishing a routine for craniotomies using the iMRI, our surgeons then approached the neuroanesthesiologists and requested that we provide awake neurocognitive testing in the iMRI suite. This request posed a new set of challenges. These challenges included our airway management choices, the head fixation device and coil, and our anesthetic management of these cases.
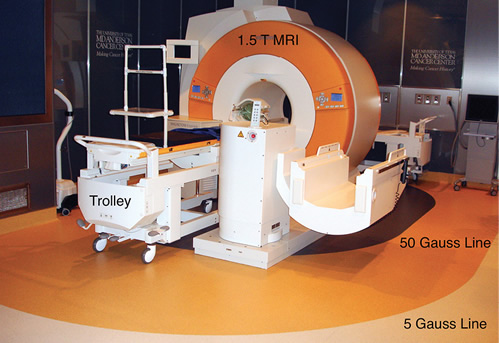
Intraoperative MRI Suite showing patient trolley, 5 gauss line and 50 gauss line.
We generally provide an asleep-awake-asleep (sedated) technique for awake craniotomies. The asleep portions of the anesthetic are usually managed with a supraglottic airway. Some of these devices contain wire reinforcement in the ventilating port and caused unwanted artifacts. Airway devices free of metallic reinforcement must be used for cases in the iMRI. The head fixation apparatus acts as the inferior coil for imaging and had to be modified to facilitate airway management during the wake up and awake periods of the craniotomy. We employ a balanced anesthetic technique utilizing propofol and remifentanil infusions. Because of the Tec 6 vaporizer incompatibility, desflurane cannot be used; therefore, our inhalational gas choices are isoflurane and sevoflurane. Our neurosurgeons readily call on electrophysiology colleagues to perform continuous motor mapping, cortical mapping, and EEG monitoring. The computers used for this monitoring are not MRI compatible and must be tethered to the walls to ensure that they do not cross the 5 Gauss line. Careful placement of all the additional wiring for successful mapping/monitoring is required to ensure that no formation of wire loops or coils occur which can result in thermal burns to the patient.
The iMRI suite was designed with a power system that can disconnect power to all but 1 power outlet designated for use by the anesthesia machine. As we expanded our practice to include right-sided awake craniotomy in the iMRI environment, we saw the need to move the anesthesia machine to be near the left shoulder of the patient. This required getting another dedicated power outlet for our use.
As technology advanced further, we were asked to study a system for laser interstitial thermotherapy in which a laser is used to thermally ablate tumors using real time MRI guidance and a fiberoptic laser transmission system. Neurosurgeons can use this technique of thermal ablation to ablate intracranial tumors deemed unresectable, as well as metastatic lesions of the vertebral column. During intracranial ablations, fiberoptic laser catheters are placed through burr holes and advanced to the tumor site with the use of the MRI. As the thermal ablation takes place, continuous MRI images show the neurosurgeon and neuroradiologist the extent of thermal injury through the tumor bed. Bony metastasis of the spine can be thermally ablated as well. The patient is positioned prone and a series of ablation catheters are placed within the vertebral bodies that contain the bony metastasis. During the ablation phase of the surgery, breath holding is needed to reduce the movement of the thorax. The laser used is a 30 W 980 nm diode laser with a fiberoptic. Since no laser energy is delivered unless the catheter is internalized, no special laser precautions are needed.
Anesthesia providers, with hearing protection in place, are required to stay in the MRI suite to control the patient’s ventilation as needed for the procedure. Since any patient movement interferes with the calculations of thermal energy, breath holds of up to 100 seconds are needed. In anticipation of the breath holds, we hyperventilate the patient on 100% oxygen for a brief period prior to the requested breath holding.
Interventional MRI
MRI has become the standard modality for assessing soft tissue. Many times a small area of concern could be identified during an MRI, but the same tissue could not be identified for biopsy using fluoroscopic or CT guidance. For these situations and also for patients with tumors located precariously close to vital structures, an interventional MRI suite was created.
The procedure room is a large room with a centrally located MRI. All of the anesthesia monitors and machines are approved for MRI use. Interventional radiologists perform these procedures. For many techniques, breath holding is required so we usually perform endotracheal general anesthesia technique. Basically the same precautions are taken as for a standard MRI scan or interventional radiology procedure.
Communication among the anesthesia providers and the specialists performing the procedure is vital and creates an additional challenge. The patient is moved into the MRI for image acquisition and then removed from the bore to place the needles. The patient is then scanned again. To provide hearing protection and also to allow communication between team members, we use wired microphone headsets/ protectors which are MRI compatible.
Proton Therapy Center
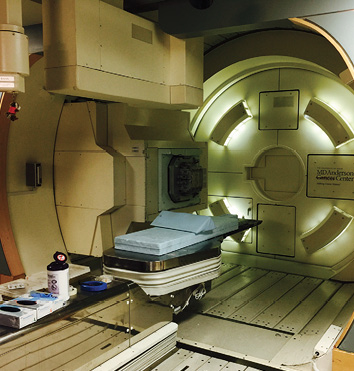
Gantry for proton beam therapy.
In 2004, MD Anderson Cancer Center in Houston opened the Proton Therapy Center. Proton therapy is the directing of a radioactive proton beam targeted to administer these protons with a precision of 1 mm. As opposed to conventional radiation therapy, the use of protons allows minimal interference of healthy tissues surrounding the tumor. This becomes particularly important for tumors located near vital organs. Tumors such as deep-seated medulloblastomas, within the brain or brainstem, were not usually amenable to standard radiation treatment using linear accelerators due to risk to surrounding tissue, but now these tumors can be treated with precision using proton therapy.
Most children under the age of 6 years require anesthesia services to prevent the dire consequences that could occur as a result of patient movement during these treatments. Our practice is currently providing about 140 anesthetics per month at the proton center. Patients requiring anesthesia services are scheduled for a CT simulation appointment and then subsequent proton therapy appointments. CT simulation appointments are required prior to the initial proton therapy appointment for the development of the proton treatment plan and immobilization devices. Immobilization devices, such as masks and cradles, are created to ensure exact repositioning of the patient for each treatment. The child is anesthetized during the simulation and a custom mask is created, which is used to immobilize the head in a predetermined position. If an airway obstruction occurs or if an oral airway or supraglottic device is required, the mask must be reformed and the simulation repeated. Masks are created from plastic net molding and holes can be created for an oxygen cannula. Properly made immobilization devices are imperative because typically proton therapy requires daily treatments for a period of 3–4 weeks.

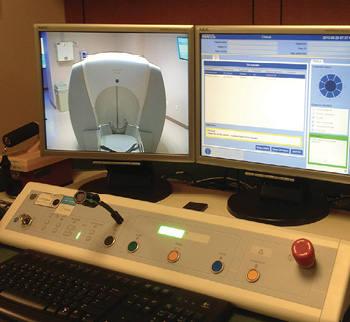
Proton Therapy Control Room.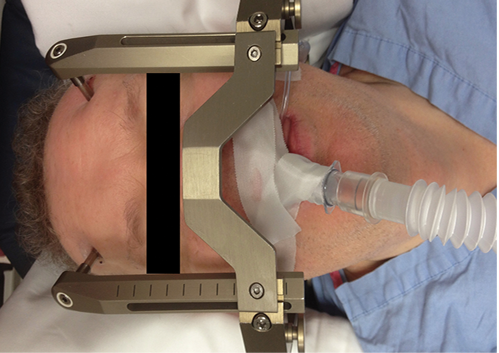 Gamma knife patient positioned within a fixed frame with an LMA in place. Availability of an appropriate Allen wrench allows emergency removal of cross bar.
Gamma knife patient positioned within a fixed frame with an LMA in place. Availability of an appropriate Allen wrench allows emergency removal of cross bar.


 Issue PDF
Issue PDF