
![]() Dear Q&A,
Dear Q&A,
This brief communication questions the current recommendations for perioperative management of cardiovascular implantable electronic devices (CIED) which include pacemakers, implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices. This case pertains to the ICD.
There are two strategies for patients undergoing surgeries who also have an internal cardiac defibrillator (ICD) to prevent inappropriate discharge of the device in the presence of electromagnetic interference (EMI). The first is to program the device to “off” by using a manufacturer’s specific programmer. The other, simpler strategy is to place a doughnut magnet directly over the devices’ generator. This will inhibit the devices’ tachyarrhythmia therapy function, not its bradyarrhythmic therapy (pacemaker) function.
The 2011 HRS/ASA expert consensus statement suggested inactivating the ICD for all surgeries above the umbilicus.1 However, the document implied that it was unnecessary to do so for surgeries below the umbilicus, as the risk of EMI being detected, and hence of discharge of the device, was very low and in fact never documented to have occurred.1 We recently cared for a patient undergoing lower extremity surgery—total knee replacement—whose ICD discharged during surgery despite appropriate placement of the electrocautery grounding pad.
The patient was an 82-yr.-old male (height: 5’ 6”, weight: 146 lbs.) with a history of coronary artery disease and an ischemic cardiomyopathy (ejection fraction=25%). The patient also had a CIED (St. Jude Medical; Little Canada, MN. Quadra Assura™ 3365-40Q CRT-D; RV lead: maximum sensitivity setting, 0.5 mV; autosense mode “on”) located in the left pectoral region. Neither a magnet nor reprogramming was used to disable the device’s antitachyarrhythmic therapy function. He subsequently underwent a right total knee replacement under spinal anesthesia. The surgery involved the use of a monopolar electrosurgical unit (ESU). The return pad was placed on the patient’s left thigh. The anesthesia-surgical course was unremarkable. However, approximately two months after his surgery, routine surveillance interrogation of the device suggested that it had discharged intraoperatively (Fig. 1 and Fig. 2, below) while functioning normally.
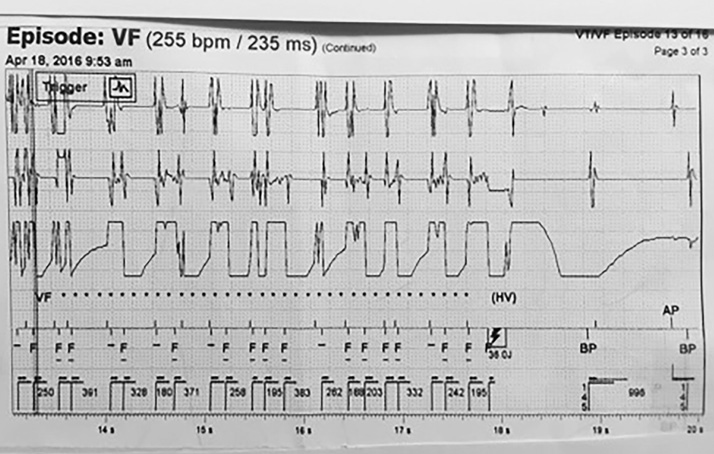
Figure 1. Top tracing: Atrial sense lead. Middle tracing: Ventricular sense lead. Bottom tracing: Discrimination (channel) lead. Atrial, ventricular, and discrimination channel leads show similar rapid wave form signals suggesting to the ICD that this is ventricular fibrillation. The device senses this and then analyzes the wave form during a programmable duration delay of approximately eight seconds. It determines that this is ventricular fibrillation, and then charges for approximately 5 seconds—while continuing to analyze the waveforms—and then discharges. The point is that the discharge event was set in motion many seconds before the actual discharge. VF *** ![]() Device charging Lightning bolt symbol indicates discharge
Device charging Lightning bolt symbol indicates discharge
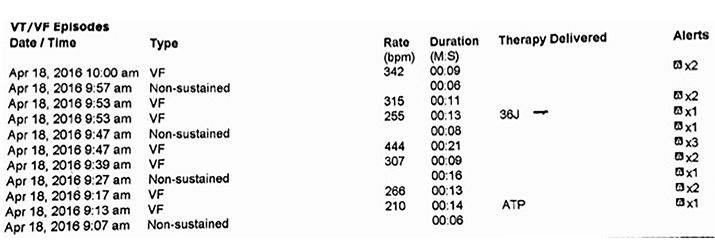
Figure 2. Table depicts events of interest, including the event leading to device discharge (electrogram shown in Fig. 1), as well as their duration. The “VF” event at 09:47 consists of several short epochs of high frequency wave forms whose total duration is 21 seconds, but with no shock delivered. The device identifies some epochs as ventricular fibrillation, but correctly identifies most as high frequency “noise” and, therefore, the device does not discharge (electrogram not shown).
In patients undergoing surgery, inactivation of the antitachyarrhythmic function of the ICD is recommended in order to prevent electromagnetic interference (EMI) from discharging the device. Unintended discharge could lead to a life-threatening arrhythmia. Two strategies are typically utilized to deactivate the device for surgeries performed above the umbilicus—either via a doughnut magnet, or programmed deactivation.2 Deactivation of the device using a doughnut magnet, however, is not foolproof. It assumes that the magnet is properly positioned and that there is appropriate contact. However, expert consensus does not necessitate using these strategies during surgery performed below the umbilicus—and particularly during lower extremity procedures—since the ICD is unlikely to sense any EMI, and therefore unlikely to inappropriately discharge. Therefore, in patients undergoing lower extremity surgery, as in our case, inactivation of the ICD via magnet application or reprogramming is not particularly recommended. In fact, the ASA Practice advisory from 2011 discourages the use of magnets in general.3 In a study comparing the efficacy of two perioperative strategies—magnet deactivation vs. program deactivation—the ICDs in the group undergoing lower extremity surgery did not record any instance of EMI.2 Our case, to the best of our knowledge, is the first documented case of ICD activation (and therefore recording of EMI) in a patient undergoing lower extremity surgery. Therefore, while the risk of ICD activation in the lower extremities is admittedly rare, it is not zero. This case calls into question prior expert consensus opinion as to the management of ICDs during surgery on the lower extremities.
Another related safety issue has arisen with the introduction of very large, nondisposable, return electrode pads (Mega Soft Dual Cord™ or Mega2000™, Megadyne Medical Products, Draper, UT) that are placed on the operating table instead of the patient. These pads are typically 36 x 20 inches for adults and utilize the principle of capacitive coupling. This large surface area for a return pad increases the risk that the ICD electrodes might be within the electric field generated by the monopolar ESU (area between the electrocautery surgical site and the return pad)even during lower extremity surgery. If the electrodes from the ICD are in the electrical field generated by the monopolar ESU, then the risk of unintentional discharge is high. This potential safety hazard was initially cited by the NHS Foundation Trust,4 but has not received wide dissemination. The increased use of the Megadyne™ return pads is another reason why the authors believe that an update to the most recent expert opinion is needed.
Magnet application over a CIED to temporarily deactivate the tachyarrhythmic therapy function (ICD) is a simple procedure. We believe that new guidelines or consensus statements should consider whether a magnet should be placed over the ICD of all patients undergoing any surgery in which a monopolar ESU will be used provided the following three caveats are met:
1) The magnet response is known and “on”;
2) the magnet is confirmed to be in the appropriate position (which in the absence of an audible tone can be difficult to determine);
3) and the magnet is in a stable position such that it will not be displaced (i.e., when patient is in the prone or lateral positions).
If these conditions cannot be met, consideration should be given to reprogram the device “off” and appropriate precautions taken. This recommendation is particularly important for patients undergoing surgery in which Megadyne™ return pads are used.
Drs. Bruce Kleinman, Sam Ushomirsky, and John Murdoch, are staff anesthesiologists at the Edward Hines Jr. VA Hospital, Hines, IL. Dr. James Loo is Chief of Anesthesiology at Edward Hines Jr. VA Hospital, Hines, IL. Jeanette Radzak is an electrophysiology nurse practitioner at Edward Hines Jr. VA Hospital, Hines, IL. Dr. Joseph Cytron is Associate Professor of Cardiology, Loyola University Medical Center.
None of the authors have any financial interest in any of the devices mentioned in the report. The opinions expressed are solely those of the authors, and are not to be construed in any way as representing the opinions of the Department of Veteran’s Affairs or the APSF.
References:
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/ American Society of Anesthesiologists (ASA) expert consensus statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: Facilities and Patient Management. Heart Rhythm 2011;8:1114–1153.
- Gifford J, Larimer K, Thomas C, et al. Randomized controlled trials of perioperative ICD management: magnet application versus reprogramming. PACE 2014;37:1219–1224.
- Practice advisory for the perioperative management of patients with cardiac implantable electronic devices: pacemakers and implantable cardioverter-defibrillators. Anesthesiology 2011;114:247–261.
- Protocol for patients with Cardiac Implantable Electronic Devices (CIEDS) undergoing surgery. Guy’s and St. Thomas’ NHS Foundation Trust. http//www.guysandstthomas.nhs.uk. (Accessed 6/15/16.)
![]() Dear Dr Kleinman,
Dear Dr Kleinman,
(From the Editors: The APSF does not have a formal position on the issue re: modification of the existing guidelines for perioperative management of CIEDs. We have recruited Dr. Streckenbach who is an expert on the perioperative management of implantable cardioverter defibrillators [ICDs] and pacemakers to provide a response to this very interesting report.)
As the number of patients with implantable cardioverter defibrillators (ICDs) presenting to the operating room for surgery has rapidly increased over the last decade, it appears that the resources readily available to help anesthesia professionals manage these devices have dwindled. Cardiologists, electrophysiologists (EP), and EP technicians along with company representatives have traditionally helped provide perioperative management of ICDs; however, the availability of this group has declined over the past several years, presumably caused by budgetary constraints. This often leaves anesthesia professionals in a difficult situation—having to manage the ICDs by themselves. There are two published guidelines that many use to help guide perioperative ICD management, the ASA Practice Advisory and the Heart Rhythm Society (HRS)/ASA Expert Consensus Statement.1-2 Both documents are very helpful, but neither can cover every scenario. In this month’s APSF publication, Kleinman et al. describe a patient who sustained an ICD shock during knee surgery despite correct placement of the bovie pad. The HRS/ASA Statement suggests that patients having knee surgery should not get shocked since “the risk of false arrhythmia detection is considered so low for surgical procedures on the lower extremities that neither reprogramming nor magnet application is considered mandatory.”1-2 Yet, Kleinman et al. reported that their patient had knee surgery and according to the data presented had an ICD shock during the surgery.
However, this shock is not exactly surprising. This author has records of two other patients (unpublished) who received unexpected shocks during hip surgery. In both cases, as in Kleinman’s, the anesthesia professionals were not aware of the shock at the time of the surgery. Kleinman’s report should certainly get everyone thinking about how to manage ICDs during lower extremity surgery. Their case is very instructive, but a quick review of how ICDs detect and treat ventricular fibrillation (VF) will be helpful before analyzing that event.
An ICD senses intrinsic R-waves through the RV sensing/pacing lead. The ICD determines the patient’s rhythm by measuring the time interval between successive R-waves. When the heart rate is 60, the interval between beats is 1000 msec (60,000 msec/60 beats). When the patient has ventricular fibrillation the heart rate is much higher—usually above 200 bpm—and the interval between beats decreases to 300 msec or less (60,000 msec/200 beats). The ICD measures each successive R-R interval and defines that interval as normal if the interval is long enough and as VF if the interval is too short. Each patient’s ICD is programmed to define what interval gets labeled as a “VF” interval. Once an ICD detects a “VF” interval, a counter starts. If enough of the subsequent intervals (for example, 12 of the next 16) meet the VF criterion, then the ICD declares the patient to be in VF. When this occurs, the ICD charges its capacitor for a shock. During charging, many ICDs will deliver anti-tachy pacing (ATP) if the HR is in a programmable range (for example 180–210 bpm). Occasionally the ATP will break the dysrhythmia obviating the need to deliver the shock. More often, however, the ATP fails and the ICD continues to charge until it is ready to deliver the shock. The charge time is approximately 4–12 seconds depending on the charge setting and the battery life. Just before delivering the first shock, most ICDs will re-assess the rhythm to confirm that the patient is still in VF. If yes, a shock is delivered. If no, the shock will be aborted, and the capacitor will slowly dissipate its charge.
It is not uncommon during electrocautery use for an active ICD to detect what it believes is VF and charge, but then abort because the cautery was not in use during the short reconfirm phase. The author is aware of cases where patients’ ICDs charge multiple times during a surgical procedure, but only deliver one or two shocks due simply to the timing of the cautery use. It is very important to understand that it takes as few as 3–4 seconds of cautery to “fool” the ICD into thinking the patient is in VF. In fact, it does not even need to be 3–4 seconds of continuous cautery—it just has to be enough bursts of cautery for the device to detect a small programmable number of very short R-R intervals. Figure 1 (see below) demonstrates how quickly cautery can be detected as VF during thoracic surgery. A magnet was used to inhibit the ICD in this case, but the magnet was displaced intermittently. Cautery was misinterpreted as VF in 3 seconds.
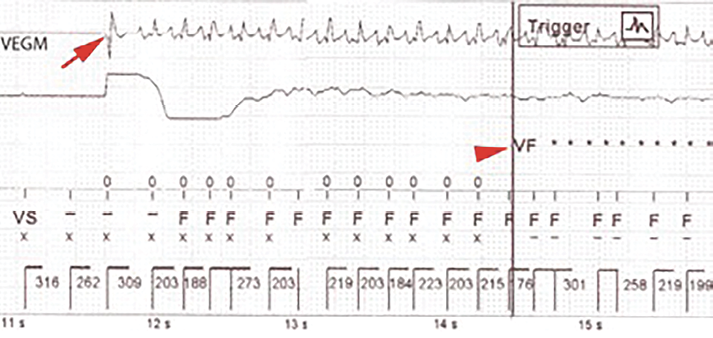
Figure 1: ICD sensing electrocautery during thoracic surgery when magnet was displaced. Arrow defines onset of cautery. Arrowhead defines detection of VF approximately 3 seconds later.
It is also worth pointing out that ICDs are more likely to sense far-field or distant cautery than pacemakers. The RV lead sensitivity setting of an ICD is high in order to detect the low amplitude fibrillation beats. Pacemaker sensitivity is lower (4–5 times typically) as pacemaker RV leads only have to detect the higher amplitude intrinsic R-waves. Thus ICDs would be more likely to sense cautery in the lower extremities than a pacer would.
To summarize, ICDs measure R-R intervals. If enough short R-R intervals occur in a short time period, the ICD detects VF and charges its capacitor. If the VF is still present when the charging ends, a shock is delivered. If it is not, the charge is aborted.
Given this simplified explanation of ICD function, the analysis of what likely transpired during the case of Kleinman et al. will be discussed. The anesthesia team followed the HRS/ASA Statement recommendations and chose to leave the ICD active for the lower extremity surgery. The electrocautery return pad was placed on the contralateral thigh. Thus cautery energy travelled from the patient’s knee up toward the waist and crossed over to the opposite leg. As electrical energy moves from its source to its destination it spreads out. How far the electrical energy spreads superiorly as it travelled to the other leg is hard to know, but my guess is that it might have gotten near the umbilicus in this particular case. The distance between the umbilicus and the RV sensing lead in this 5’6” patient is approximately 6 inches. Since the sensitivity of the ICD’s RV sensing lead is high (and thus the required amplitude of the signal required is low), the low amplitude electrocautery signal was presumably detected as if it represented intrinsic R-waves. Since the frequency at which these “R-waves” were occurring was high, the ICD quickly detected enough short R-R intervals to fulfill the VF criterion. In fact, this happened 7 times between 9:13 am and 10:00 am (see Figure 2 in the report by Kleinman et al.). While charging ensued during the 9:13 am episode, ATP was delivered by the ICD. ATP was delivered only in the 9:13 am episode because the detected average HR was above the ATP range in the other VF episodes. The anesthesiologist might have noticed a series of rapid pacing spikes with paced R-waves, but only if he or she had been looking at the monitor at this time, and if cautery were not being used. In that same episode, when the charging completed, the ICD did not sense the electrocautery so the shock was aborted. Shortly thereafter, during a 9:53 am VF episode, the ICD confirmed VF at the end of charging, and shocked the patient with 36 joules.
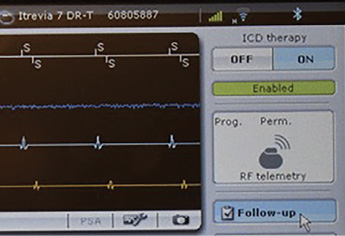
Figure 2: Biotronik ICD programmer screen upon initial interrogation depicting one-touch anti-tachy disabling option.
In Figure 1 (see top of article) of Kleinman et al.’s report, notice the electrocautery signals are being detected as VF intervals (the “F”s). Charging occurred coincident with the asterisks. It took approximately 5 seconds for the charge (this duration was likely shortened by the prior charge without complete charge dissipation at 9:47 am). When charging completed at the last asterisk, the next interval (195 msec) says “F” indicating that cautery was detected at that moment, and the ICD presumed that the dysrhythmia persisted. It therefore delivered the 36-joule shock to a heart presumably in sinus rhythm. The anesthesiologist might have noticed this shock depolarization on the EKG if he or she were looking at the monitor devoid of cautery noise, but it would have been very short in duration. The patient who had a spinal most likely would have moved somewhat, the intensity dependent on the patient’s muscle mass. Again the anesthesiologist may have been charting and did not notice it—or could have thought it was a cough. The surgeons too may have noticed it but presumed the motion to indicate a cough or just random patient movement.
An ICD misinterpreting cautery as VF is problematic for several reasons. First, ATP or a high voltage asynchronous shock, during sinus rhythm, can actually induce ventricular fibrillation. Second, the ICD battery can be significantly depleted. Each shock can diminish the battery by an estimated 30 days according to manufacturer technical support staff. Moreover, a charge even without a shock diminishes battery life. There is a report of total ICD battery depletion during a surgical procedure related to this issue.3 Third, patient movement from a shock during a critical moment could cause a surgical complication. Finally, there is evidence that shocks, appropriate or inappropriate, cause myocardial injury and increase mortality.4
So what should the readers do with the knowledge of the case presented by Kleinman et al.? I think readers should understand that whether an ICD will sense electrocautery or not depends on more than just the location of the surgery. It significantly depends on the location of the electrocautery return pad. If the return pad can be placed on the ipsilateral leg (e.g., on the left calf during left hip surgery) the likelihood of an ICD detecting the cautery is very low—consistent with the statement made in the HRS/ASA Consensus Statement. However, if the return pad is placed on the contralateral leg, especially in a small patient, one may have to consider the risk of an inappropriate shock to be higher than suggested by the Consensus Statement. Certainly, if the return pad is placed on the patient’s back, the risk of an inappropriate shock is very high. Finally, I think it is also important to remember that some OR nurses may not be so acutely aware of the relevance of the position chosen to place the electrocautery return pad. Certainly if you plan to leave an ICD on during a surgical procedure that will include electrocautery, you should discuss the placement of the return pad preoperatively with the circulating nurse.
In the second half of this paper, I will make a few recommendations intended to improve the anesthesia professional’s ability to manage patients who have ICDs. First, I recommend that you read the HRS/ASA Consensus Statement in its entirety if you have not already. It provides excellent electrophysiology education as well as guidance for perioperative device management. It has concise summaries of the questions you should ask about the patient’s device, and it includes charts that describe the function of both ICDs and pacemakers. Whenever colleagues ask what they should read to learn more about perioperative electrophysiology, I always tell them to start with this paper.
Second, I recommend that you become a magnet specialist if there is any chance you will ever choose to use a magnet in the OR. In other words, I suggest that you learn in great detail exactly how ICDs from each of the manufacturers interact with a magnet. Table 1 (see below) provides my summary of these magnet-ICD interactions. You should notice that a properly placed magnet will inhibit the anti-tachy therapy of all ICDs. The only possible exception to this statement would be a very rare Boston Scientific or St. Jude ICD that was programmed to ignore a magnet—see below. When the magnet is removed from any of the ICDs, the anti-tachy therapy resumes.
Next, notice how a magnet affects the pacemaker component of each ICD, remembering that all ICDs have an integrated pacemaker. A magnet is ignored by the pacer component of every brand of ICD except for Sorin (the base rate increases). In no situation will a magnet convert an ICD’s integrated pacer to an asynchronous pacing mode as a magnet routinely does with stand-alone pacers. The only way to make the ICD’s pacer asynchronous is to re-program the ICD using a company-specific programmer.
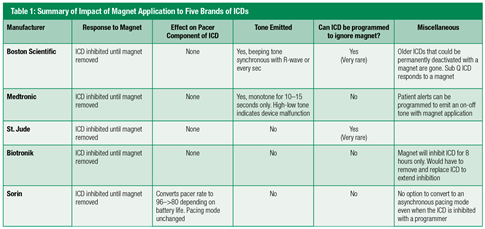
Next, notice that ICDs differ in the tone each emits when a magnet is applied (see Table 1, above). It is very important that you understand these differences. Boston Scientific ICDs emit a beeping tone (every second or coincident with the R-wave) for as long as the magnet is on the ICD. This tone can be heard even in the noisy operating room by applying one’s stethoscope over the hole in the magnet. This tone can help confirm the proper magnet location throughout an entire procedure. Medtronic ICDs emit a tone that lasts for 10–15 seconds after placement of a magnet. A continuous 10–15 second tone indicates that the anti-tachycardia component of the ICD is inhibited. The Medtronic tone can at least confirm your initial magnet placement, but it does not facilitate intermittent checking during the procedure. The other devices (St Jude, Biotronik and Sorin) do not emit a tone. This means that one cannot confirm that the magnet is properly positioned initially or throughout the entire surgical procedure with these latter three ICDs. This issue is particularly worrisome if the patient is obese and the ICD is difficult to palpate, or if the patient is in a lateral or steep Trendelenburg position. The author has 5 records of patients whose ICDs were presumably inhibited with a magnet during a procedure who nevertheless got intraoperative shocks. Two of these patients were in the lateral position and one was obese.
Finally notice, as mentioned above, that ICDs differ in their ability to potentially ignore a magnet. Boston Scientific and St Jude ICDs can be programmed to ignore a magnet; Medtronic, Biotronik and Sorin ICDs cannot. Thankfully, ICDs are very rarely programmed to ignore a magnet, but one should always confirm that the ICD would respond to a magnet before the surgery begins. In my experience, the typical EP or cardiology follow-up note does not define this magnet response. If your patient has a Boston Scientific device, you can place the magnet over the ICD, and if you hear beeping tones, you can be assured that the ICD is responding to the magnet and therefore will inhibit the ICD’s anti-tachy therapy. To know for certain that a St. Jude ICD will respond to a magnet, you will need to get that specific information from the patient’s cardiologist, or you will need to use a programmer to interrogate the device.
Anesthesia professionals who choose to use a magnet to inhibit an ICD perioperatively certainly should know precisely how the ICD will respond to a magnet. They must be very careful to ensure that the magnet position relative to the ICD is appropriately maintained. Defining the border of the ICD with a marking pen before securing the magnet over the ICD makes periodic monitoring easier. They should also remember that if a patient develops VF while the ICD is inhibited with the magnet, they might still want to use an external defibrillator. If they choose to remove the magnet so that the ICD can treat the dysrhythmia, it will take 3–4 seconds to detect the VF and another 5–10 seconds for the ICD to charge before the shock can be delivered. Watching VF for up to 15 seconds in the OR waiting for the internal ICD to shock the patient is not ideal.
My last recommendation intended to improve the ability to manage patients with ICDs relates to device programmers. Although there are few of them, trained anesthesia professionals can use a programmer to disable the ICD’s anti-tachy therapy. Each company’s programmer is different, but it is still relatively easy to turn off the anti-tachy therapy, especially for Boston Scientific, St Jude, and Biotronik ICDs. In fact, only one step is required to turn off the anti-tachy therapy of a Biotronik ICD (Figure 2). Manufacturing representatives are very skilled at training anesthesia professionals on how to use their programmers and appear willing to provide a programmer for perioperative use. With practice, typically with EP colleagues, motivated anesthesia professionals should be able to learn very basic programming such as suspending anti-tachy therapy and changing pacing modes.
Disabling the ICD’s anti-tachy function with a programmer prevents inappropriate shocks and obviates the need to use a magnet during a procedure. This reprogramming is a good alternative to using a magnet especially in obese patients, or those who will not be supine throughout the procedure. Using the programmer also provides the option to change the pacing mode of an ICD when indicated. In fact, one has to use the programmer in order to convert the pacemaker function of an ICD to asynchronous pacing (e.g., DOO). An asynchronous pacing mode is only programmable after the anti-tachy therapy is suspended with the programmer.
A downside of reprogramming the ICD’s anti-tachy therapy is that the device will not be readily able to treat an intraoperative dysrhythmia. Therefore, cardiac monitoring and immediate availability of external defibrillation are essential once the ICD is suspended. Also, the ICD must be reactivated prior to the patient being discharged from the hospital. The advantage of anesthesia professionals doing the programming is that they can typically turn off the ICD when the patient is already in the OR and fully monitored. As soon as the surgery is done, an anesthesia professional is typically available to reactivate the ICD before the patient goes to the recovery room. This process minimizes the time during which the ICD is off and decreases the risk of a patient getting discharged with the ICD suspended. This is exactly how I manage the majority of the patients at my hospital.
Anesthesia professionals who understand basic electrophysiology and know how to utilize the device programmers may be able to effectively manage CIEDs in the perioperative period. The anesthesia professional can be aware of the cautery needs, the positioning issues, the ability to use a magnet based on the surgical site, and the patient’s medical history. More importantly, the anesthesia professional is in the OR with the patient. A recently published article demonstrated that anesthesia-driven care of CIEDs can be safe and time saving.5
Obviously not every anesthesia professional can be expected to learn how to use a programmer. However, hospitals could support the training of a few motivated individuals who could then develop the framework necessary for such an endeavor. Professional organizations for anesthesia providers could develop online training programs that could be used by anesthesia professionals who have less access to manufacturing representatives and EP services. At the MGH, we are in the middle of this training process, closely collaborating with our EP service and its rewards for patient safety and throughput are abundantly clear.
Dr. Scott Streckenbach, is presently Assistant Professor, Harvard Medical School and Director of Perioperative Electrophysiology in the Division of Cardiac Anesthesia and in the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital.
Disclosure: Dr. Streckenbach reports no financial conflicts of interest for this article.
Bibliography
- American Society of Anesthesiologists. Practice Advisory for the perioperative management of patients with cardiac implantable electronic devices: pacemakers and cardioverter-defibrillators: an updated report. Anesthesiology 2011:114:247–61.
- Crossley GH, Poole JE, Rozner MA et al. The Heart Rhythm Society Expert Consensus Statement on the perioperative management of patients with implantable defibrillators pacemakers and arrhythmia monitors. Heart Rhythm 2011;8:1114–53.
- Schulman PM and Rozner MA. Use caution when applying magnets to pacemakers or defibrillators for surgery. Anesth Analg 2013;117:422–7
- Moss AJ, Schuger C, Beck CA et al. Reduction in inappropriate therapy and mortality through icd programming. N Engl J Med 2012;367:2275–83.
- Menzel Ellis MK, Treggiari MM, Schulman PM, et al. Process improvement initiative for the perioperative management of patients with a cardiovascular implantable electronic device. Anesth Analg 2017 Mar 17 electronic publication ahead of print.
The APSF sometimes receives questions that are not suitable for the Dear SIRS column. This Q and A column allows the APSF to forward these questions to knowledgeable committee members or designated consultants. The information provided is for safety-related educational purposes only, and does not constitute medical or legal advice. Individual or group responses are only commentary, provided for purposes of education or discussion, and are neither statements of advice nor the opinions of the APSF. It is not the intention of the APSF to provide specific medical or legal advice or to endorse any specific views or recommendations in response to the inquiries posted. In no event shall the APSF be responsible or liable, directly or indirectly, for any damage or loss caused or alleged to be caused by or in connection with the reliance on any such information.


 Issue PDF
Issue PDF