This issue of the APSF Newsletter sheds new light on an old issue and challenges clinicians to refocus their attention on health care-associated infections (HCAI) and even more relevant, surgical site infections (SSI). Infection control practices that were appropriate for the anesthesia work environment at the middle and end of the 20th century are largely irrelevant today as medical, technical, environmental, and microbiological challenges are infinitely more complex and far less predictable than the operating room of the 1960s. New and thought-provoking recommendations for anesthesia professionals are summarized in a recent seminal publication by the Society for Healthcare Epidemiology of America (SHEA).1 This guidance was generated by 15 individuals with expertise in this field, representing input from the American Society of Anesthesiologists (ASA), American Association of Nurse Anesthetists (AANA), American Academy of Anesthesiologist Assistants (AAAA), American College of Surgeons (ACS), SHEA, and others.1 This expert compendium issues guidance on how hospitals and health care providers may reduce infections associated with anesthesiology procedures and equipment in the operating room and highlights the importance of improved hand hygiene, increased environmental disinfection, and safer medication injection practices.
Why is there concern for this issue?
Two million hospitalized patients develop HCAI annually, contributing to over 90,000 deaths each year in the United States.2 The source of these infections is multifactorial, but there is increasing evidence that a significant fraction of these infections originate while patients are in the operating room—and routine anesthesia practices may contribute.3,4 Alarmingly, a survey of 49 U.S. and international facilities as part of the SHEA guidance showed infection control policies and practices for providers are generally inconsistent, misunderstood, or nonexistent.1
However, some in the anesthesia community question if the issue of anesthetic practice contributing to HCAI is real. Two factors likely contribute to this misunderstanding: the “fecal patina” (coating of enteric organisms that are on patient’s skin and on surfaces in the health care environment that are contacted by patients and health care professionals in the operating room) is invisible3 and difficult to sterilize, and most SSI infections present several days after surgery. Meanwhile, there is no debate about the profound consequences of HCAI that include increased costs, selection pressure for drug-resistant organisms, patient and family dissatisfaction, significant morbidity and mortality, and potential liability. Surgical site infections are especially relevant as they account for 20% or more of all HCAI. Indeed, SSIs afflict up to 3% of all surgical patients (depending on the type of surgery, patient co-morbidities, length of operation, etc.) increasing the hospital length of stay from 3 to 10 days and increasing mortality 2- to 10-fold.2
How can anesthesia practices contribute to HCAI? Poor hand hygiene is a primary suspect. Observed risk factors for poor hand hygiene include status as a physician, working as an anesthesia professional, short duration of care, and interruption in patient care activities.3,4 A recent study also identified bacterial contamination of drugs and drug syringes during routine administration of anesthesia in the operating room.5 Over 6% of microbial filters placed in standard IV tubing of anesthetized patients were contaminated with Staphylococcus, Corynebacterium, and Bacillus species.5 Equally alarming, 2.4% of fluid samples from the residual drug within syringes at the end of surgical cases grew these same and additional organisms.
What can be done? The SHEA document promotes several key recommendations
Hand hygiene should be performed, at a minimum, before aseptic tasks, after removing gloves, when hands are soiled, before touching the anesthesia cart, and upon room entry and exit. Every anesthetizing site should have strategic placement of alcohol-based hand sanitizer dispensers.
- The interactions between anesthesia professionals and operating room equipment, the anesthesia machine, monitor surfaces, computers and keyboards, vascular catheters, stopcocks, and intravenous tubing were documented during eight hours of operating room observation in a recent study.6 Anesthesia providers, on average, touched these surfaces 1,132 times, completed 66 stopcock injections, and inserted four vascular catheters.6 Unfortunately, appropriate hand hygiene preceded only a small fraction of these actions.
As part of airway management, clinicians need to use high-level disinfection of reusable laryngoscope handles or adopt single-use laryngoscopes.
- Flexible and rigid larynoscopes—both blades and handles—are classified as semicritical devices (because they contact mucous membranes), and therefore require both cleaning and “high-level disinfection or sterilization.” Medical literature documents outbreaks of virulent organisms like Pseudomonas aeruginosa attributed to dirty laryngoscopes. Moreover, many institutions are discovering that the cost of reprocessing reusable laryngoscopes to this new standard is substantial.7 While cost allocation data depend on your specific organization, adopting single-use products may actually be quite cost favorable. Table 1 compares several aspects of these two laryngoscope options.7
Table 1: Infection and Larygnoscopes: Comparison of Reusable and Single-Use Laryngoscopes7
| Traditional, Reusable Laryngoscopes | Single-Use Disposable Laryngoscopes |
| Batteries wear out, need replacement | Batteries always brand new |
| Bulbs dim and eventually burn out | Light source always new |
| On-off switch prone to wear and failure | Switch is new; testable while still in package |
| Handles require disassembly to disinfect | No cleaning or maintenance of device |
| Requires sterilization or high-level disinfection after each use | Provided sterile in new, transparent package |
| Costs rise rapidly with newly required processing and sterilization | Costs at parity or even less expensive depending on the institution |
| Performance is well known with a familiar feel | Performance now usually rated at parity with reusable laryngoscopes |
| With permission to reuse from Prielipp RC, Birnbach DJ. APSF Newsletter. 2018;32:65. https://www.apsf.org/article/hca-infections-can-the-anesthesia-provider-be-at-fault/ Accessed August 13, 2019. | |
For environmental disinfection, the guidance statement recommends disinfecting high-touch surfaces on the anesthesia machines, as well as keyboards, monitors and other items in work areas in between surgeries, while also exploring the use of disposable covers and re-engineering of the work surfaces to facilitate quick decontamination in what is often a short window of time.
- Surfaces in a typical operating room are likely to grow pathogens such as MRSA, VRE, MSSA, E. coli, and Acinetobacter even after routine room cleaning. Decontamination of the environment becomes critical as additional evidence highlights that the probability of bacterial growth in injection stopcocks is a function of the number of bacterial colonies contaminating the anesthesia machine as well as baseline hand contamination of anesthesia professionals.3,4
- In addition, contamination of multiple clean OR surfaces occurs rapidly and in wide distribution around the anesthesia workplace following intubation and airway management. Of particular alarm, a simulation study demonstrates 100% contamination of the IV hub, anesthesia circuit, and anesthesia cart within six minutes of induction and endotracheal intubation of patients.8 Moreover, there is compelling evidence of contamination of unused syringes placed on the work surface of the anesthesia cart or machine, suggesting that all syringes (even if unused) be discarded at the end of each case.8
IV drug injection recommendations include using syringes and vials for only one patient; and that injection ports and vial stoppers should only be accessed after disinfection.
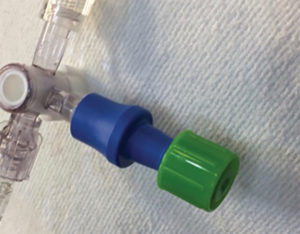
- Stopcocks should preferentially be converted to “closed injection ports”, or, if not being used immediately to inject medications, should at least be covered with sterile caps (see Figure 1).
Conclusion
“Clean Care is Safer Care” is not a choice but a basic right. Clean hands prevent patient suffering.”
—World Health Organization
The reality is that health care providers who work in the OR are subject to the inevitable variability of human performance, both individually and collectively. In addition, the motivation of health care workers to adopt new, safer—but more demanding—interventions such as those detailed in the SHEA guidelines is often counteracted by instincts to simply maintain old, familiar, and “comfortable” habits. Common reasons for this are fear of the unknown, work overload, scientific uncertainty, and lack of individual and organizational adaptability. Last but not least, production pressure in most OR situations prioritizes efficiency rather than being thorough. Indeed, safety management characterizes this principle with the acronym ETTO—the efficiency-thoroughness trade-off.9 The ETTO fallacy is that people can always be simultaneously be both efficient and thorough at the same time.
In summary, we encourage anesthesia professionals to embrace these new principles, practices, and opportunities to improve patient care. The SHEA guidance and similar algorithms are a starting point. In the words of the 18th century physicist Georg Lichtenberg, “I cannot say whether things will get better if we change; what I can say is they must change if they are to get better.” We hope these SHEA guidelines will tip the balance in favor of thoroughness and safety for every patient, every case, every time as we again lead the medical community in patient safety.
Dr. Richard C. Prielipp is professor of Anesthesiology at the University of Minnesota in Minneapolis and serves on the speaker’s bureau for Merck & CO., Inc. He is a consultant for Fresenius Kabi, the executive section editor, Patient Safety for Anesthesia & Analgesia, and is on the APSF Board of Directors.
Dr. Birnbach is Miller Professor of Anesthesiology, and director, UM-JMH Center for Patient Safety, University of Miami.
Drs. Prielipp and Birnbach served as members of the taskforce for the development of the SHEA Guidelines.
References
- Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area. Infect Control Hosp Epidemiol. 2018;11:1–17.
- Davis CH, Kao LS, Fleming JB, et al. Multi-institution analysis of infection control practices identifies the subset associated with best surgical site infection performance: A Texas Alliance for Surgical Quality Collaborative Project. J Am Coll Surg. 2017;225:455–464.
- Munoz-Price LS, Weinstein RA. Fecal patina in the anesthesia work area. Anesth Analg. 2015;120:703–705.
- Loftus RW, Muffly MK, Brown JR, et al. Hand contamination of anesthesia providers is an important risk factor for intraoperative bacterial transmission. Anesth Analg. 2011;112:98–105.
- Gargiulo DA, Mitchell SJ, Sheridan J, et al. Microbiological contamination of drugs during their administration for anesthesia in the operating room. Anesthesiology. 2016;
124:785–794. - Munoz-Price LS, Riley B, Banks S, et al. Frequency of interactions and hand disinfections among anesthesiologists while providing anesthesia care in the operating room: induction versus maintenance. Infect Control Hosp Epidemiol. 2014;35:1056–1059.
- Prielipp R, Birnbach D. HCA-Infections: Can the anesthesia provider be at fault? APSF Newsletter. 2018; 32: 64–65. https://www.apsf.org/article/hca-infections-can-the-anesthesia-provider-be-at-fault/ Accessed August 13, 2019.
- Birnbach DJ, Rosen LF, Fitzpatrick M, et al. The use of a novel technology to study dynamics of pathogen transmission in the operating room. Anesth Analg. 2015;
120:844–847. - Hollnagel E. Safety-I and Safety-II. The past and future of safety management. Ashgate Book, CRC Press. New York, 2014.


 Issue PDF
Issue PDF