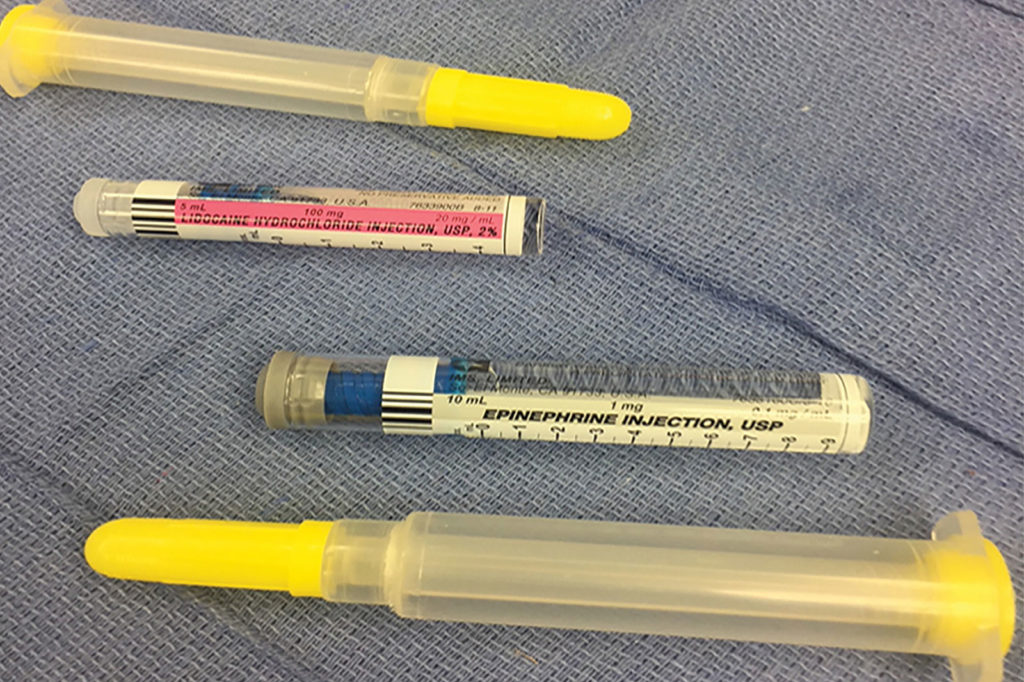
Around the time of the publication of the letter “‘No Read’ Errors Related to Prefilled Syringes” in this publication,1 we experienced a similar incident in our institution. Use of prefilled syringes has been found to have many advantages including convenience, sterility, and safety.2 In this case, prefilled syringes of epinephrine 0.1 mg/ml and lidocaine 2% were assembled prior to induction of anesthesia and placed on top of the anesthesia cart (Figure 1A and 1B). Both syringes were manufactured by IMS, Limited of South El Monte, CA. For induction, syringes were placed into the intravenous manifold by a beginning anesthesia trainee. The trainee called out what medication was being administered as the trainee administered the drugs. The trainee stated that 100 mg of lidocaine was being administered. Shortly after induction the patient became very hypertensive and tachycardic. When the anesthesia attending looked at the drugs in the manifold, it became apparent that 0.8 mg of epinephrine had been administered instead of lidocaine. Propofol and esmolol were given to counteract the effects of the epinephrine, which resulted in profound hypotension. Low-dose epinephrine was given and a few chest compressions were administered. The patient quickly stabilized and the case proceeded as scheduled. There were no ECG changes and associated cardiac enzymes were negative.
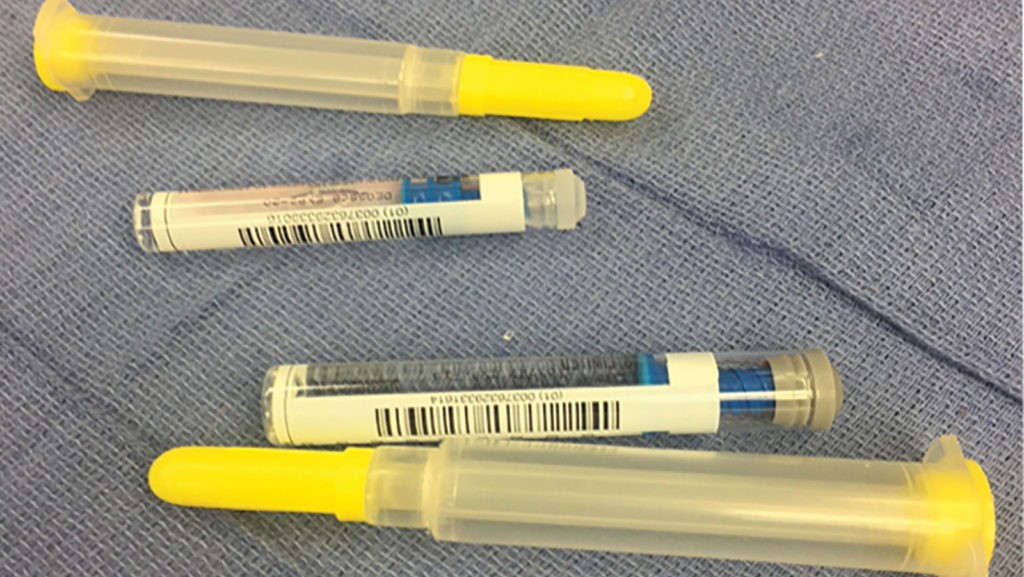
Figure 1B: Depicts the back labeling of the 2% lidocaine and epinephrine 0.1 mg/ml syringes. Note the similarity between the syringes.
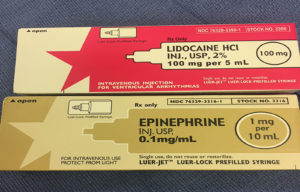
The case was presented to the hospital quality improvement committee and it was determined that the syringes, when assembled, looked very similar and that color coding of the syringes did not meet standards set by the American Society for Testing and Materials (ASTM).3 In fact, the lidocaine syringe and box have a pink label, which is close to the violet used for vasopressors in ASTM standard labeling (Figure 2). In addition, the epinephrine has a grey/tan label, similar to the grey used in the ASTM standards for local anesthetics (Figure 2). Neither has circumferential color labeling (Figure 1).
As a result of this incident, the following institutional policies were implemented:
- Epinephrine syringes are not to be assembled until they are needed.
- Epinephrine is not to be kept on top of the anesthesia cart.
- Beginner trainees are not to administer induction drugs.
We hope these interventions will reduce the risk of medication error and subsequently improve patient safety.
Gage Parr, MD, FASA, is director of Quality Improvement and assistant professor at the Department of Anesthesia and Critical Care Medicine, George Washington University Hospital, Washington, DC.
Tricia Desvarieux, MD, is assistant professor, Department of Anesthesia and Critical Care Medicine, George Washington University Hospital, Washington, DC.
Daniel Fisher, MD, is anesthesia resident, Department of Anesthesia and Critical Care Medicine, George Washington University Hospital, Washington, DC.
The authors have no conflicts of interest to disclose.
References
- Hand W, Cancellaro V. “No Read” Errors related to prefilled syringes. APSF Newsletter. 2018;33: 20–21. https://www.apsf.org/wp-content/uploads/newsletters/2018/june/pdf/APSF201806.pdf Accessed August 19, 2019.
- Makwana S, Basu B, Makasana Y, et al, Prefilled syringes: an innovation in parenteral packaging. Int J Pharm. 2011;1:200–206.
- ASTM D4774-11e1. Standard specification for user applied drug labels in anesthesiology. Available at: https://www.astm.org/Standards/D4774.htm Accessed August 8, 2019.


 Issue PDF
Issue PDF