Introduction:
 Most preventable adverse events in medicine are caused by communication errors and more than half of these occur in relation to care transitions.
Most preventable adverse events in medicine are caused by communication errors and more than half of these occur in relation to care transitions.
Hudson et al. found a 43% increase in in-hospital mortality and a 27% increase in major morbidity associated with intraoperative handoffs between anaesthetists in cardiac surgery.1 Saager et al. and
Hyder et al. both observed a similar effect during non-cardiac surgery.2, 3
 In recent years, there has been an increasing focus by The Joint Commission on improving handoffs.4 This could be in part the result of a reduction in resident duty hours, resulting in increasing numbers of providers taking care of patients in any given time period5 coupled with a perceived lack of education and the known risks of communication errors.
In recent years, there has been an increasing focus by The Joint Commission on improving handoffs.4 This could be in part the result of a reduction in resident duty hours, resulting in increasing numbers of providers taking care of patients in any given time period5 coupled with a perceived lack of education and the known risks of communication errors.
Handoff checklists are an easy way to standardize oral communication and to reduce the loss of information. Several well-designed studies have shown their positive effect on postoperative handoffs by anesthesia professionals to PACU nurses,6-8 but the intraoperative period has received much less attention.9,10 We recently implemented an intraoperative communication module (consisting of a training module and a communication checklist) in the OR at a large tertiary care University Hospital11 and would like to share some important lessons learned from that experience, most of which incorporate the basic steps for any change in management for new program implementation.12
From Planning to First Draft
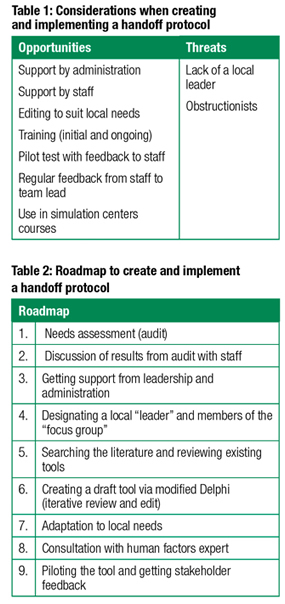
There are a number of considerations when creating the implementation of a handoff protocol (see Table 1). A good starting point (see Table 2) is some form of an audit (ideally by an anonymous observer—anonymous to avoid the Hawthorne effect) to assess current practice. The results of the audit could form a basis for initial discussions with staff. This process would identify documenting deficiencies, obtain support, and begin the planning process for developing a handoff tool. Support from leadership and administration is critical and will likely be a strong predictor for successful long-term implementation.
The next step in planning is to recruit a local “leader” and a small group of interested individuals who would become part of a working group to design and implement the tool in collaboration with the local stakeholders. As the project evolves (often starting with a review of the existing literature and other existing tools), a first draft is constructed. The construction may employ the Delphi method, an iterative review/edit process that will give the final product some validity. It will be important to adapt the tool for local needs and conduct a pilot trial. This testing can be done either in a simulation center, through real-life practice sessions, or via live observations. Simulation has the advantage of allowing the testing to occur under stressful conditions (which could reveal important hidden shortcomings in the cognitive tool design). Feedback from the users will then have to be addressed. Depending on the complexity of the protocol tool and the available resources, a consultation with a human factors consultant may help to optimize the design. Outside of academic centers, this may require hiring an external consultant.
The development process will likely require several rounds of revising the draft tool in consultation with stakeholders (including administration and leadership) until a first workable version will be ready.
Implementation
Dedicated in-service and training sessions are important steps in providing the stakeholders with the necessary tools for successful use. Even though many checklists (especially for emergencies) are intended to be intuitively easy, the lack of training can negatively impact successful use.13 Several initial in-service and training sessions (as well as some ongoing refreshers) will be necessary to ensure that all staff have been trained and to reassess the full benefit of the tool. Because it may be difficult to have a control group for a comparison of tool performance after the implementation, it may be most practical to perform such an assessment using a “Before-and-After” study design.14
The Role of Computing Technology
The increasing use of Electronic Medical Records (EMRs) and Automated Anesthesia Information Systems (AIMS) may make it necessary to contact the local vendor via the hospital administration to explore options for integration of the handoff tool in the available EMR/AIMS (or at the very least, to ensure appropriate documentation of its use). Since several major vendors now already have their own handoff tools, the options for possible customization will have to be discussed on a case-by-case basis.
Creation of a Handoff Application
The presentation of a cognitive aid can take on several forms (as simple as a laminated card, posted in a strategic location, an electronic version on the hospital’s intranet, or a smartphone application, etc.). Creation of an application deserves special consideration and will be discussed further. The market for mobile health care applications (app; most commonly Apple’s® iTunes Store and Google’s® Google Play) continues to expand exponentially and is changing the way health care professionals (and also patients) access and manage information. Recent estimates suggest that there may now be over 150,000 applications available with several thousand appearing every year.15
Mobile health applications, “apps,” are generally defined as a software application specifically designed for a smartphone or hand-held device that enables the app to receive and communicate health-related services or data (often with some degree of computation). Medical apps that diagnose, manage, and treat disease are regulated as medical devices and therefore are associated with a number of complex regulatory challenges that have attracted the attention of the FDA. The FDA published a statement clarifying that it intends to apply its regulatory authority only to mobile applications whose functionality could “pose a risk to a patient’s safety if the mobile app were to not function as intended.” It appears that the FDA will primarily target applications that communicate with sensors (for example an application that can obtain an ECG rhythm and diagnose atrial fibrillation, such as Kardia®) as opposed to applications that merely present information that is published elsewhere (such as ASRA Coags).16
If the application (such as the mere visual display of a common checklist) does not provide decision-making support and does not store any patient information (the latter of which could prompt concerns with the privacy of health information), then it will likely not require FDA approval as a “medical device.” It is, however, advisable to seek input from a qualified lawyer, who would also assist in drafting the appropriate disclaimer that any user would have to accept before using the application. Since some medical malpractice carriers may not cover non-patient-related activities (such as administration and research; educational activities; creative professional activities such as publications and app development), an insurance policy against potential litigation should be considered (personal communication CMPA—Canadian Medical Protective Association, 2016). Different procedures and policies may apply to other countries. An additional strategy to limit personal liability for the developer/owner of the app may be to create a corporation that owns the app.
Writing the code for an application requires special expertise and is usually outside the scope of practice for most clinicians. It may become necessary to contract a reputable app developer to discuss the project and get an estimate for the app design, testing, and publication (and a special allowance should be calculated to allow changing of certain features as the development progresses). The cost for a simple communication tool application17 is in the order of about $15,000 (not including the cost of time of the involved clinicians). Additional costs would be incurred to cover future updates to maintain functionality with updates to the platforms’ operating systems and to keep the content of the app in sync with evolving clinical practice. The costs for more complex application will obviously increase with the time required—for example the development of the pediatric crisis checklist “Pedi Crisis” required over 2,000 person-hours of physicians, computer scientist and software engineers.18
Summary
Handoff training and the availability of cognitive aids (posting of printed handoff checklists in each OR, computerized checklists, integration with EMR or AIMS, smartphone applications etc.) have the potential to improve the quality of communication during an intraoperative transfer of care. Education and training are a cornerstone of such a process and significant resources are required.
Dr. Kurrek is presently Professor of Anesthesiology at the University of Toronto, Toronto, Canada.
Dr. Kurrek has developed ‘AnesList’ on AppStore and Google Play. He received no support and the app is downloadable for free. He derives no monetary profit from it.
References
- Hudson CC, McDonald B, Hudson JK, et al. Impact of anesthetic handover on mortality and
morbidity in cardiac surgery: a cohort study. J Cardiothorac Vasc Anesth 2015;29:11–6. - Saager L, Hesler BD, You J, et al. Intraoperative transitions of anesthesia care and
postoperative adverse outcomes. Anesthesiology 2014;121:695–706. - Hyder JA, Bohman JK, Kor DJ, et al. Anesthesia care transitions and risk of postoperative
complications. Anesth Analg 2016;122:134–44. - The Joint Commission (http://www.centerfortransforminghealthcare.org/projects/detail.aspx?Project=1
(last accessed 20170531). - Nasca TJ, Day SH, Amis ESJ. The new recommendations on duty hours from the ACGME Task Force.
N Engl J Med 2010; 363:e3. - Weinger MB, Slagle JM, Kuntz AH, et al. A multimodal intervention improves postanesthesia
care unit handovers. Anesth Analg 2015;121:957–71. - Segall N, Bonifacio AS, Schroeder RA, et al. Can we make postoperative patient handovers
safer? A systematic review of the literature. Anesth Analg 2012; 115:102–15. - Salzwedel C, Bartz HJ, Kühnelt I, et al. The effect of a checklist on the quality of
post-anaesthesia patient handover: a randomized controlled trial. Int J Qual Health Care 2013;25:176–81. - Agarwala AV, Firth PG, Albrecht MA, et al. An electronic checklist improves transfer and
retention of critical information at intraoperative handoff of care. Anesth Analg 2015;120:96–104. - Boat AC, Spaeth JP. Handoff checklists improve the reliability of patient handoffs in the
operating room and postanesthesia care unit. Paediatr Anaesth 2013; 23:647–54. - Jullia M, Tronet A, Fraumar F, Minville V, et al. Training in intraoperative handover and
display of a checklist improve communication during transfer of care. Eur J
Anaesthesiol 2017;34:1–6. - Gesme D and Wiseman M. How to implement change in practice. J Oncol Pract 2010;
6:257–259. - Hilton, G, Daniels, K, Carvalho, B. Simulation study assessing healthcare providers knowledge
of pre-eclampsia and eclampsia in a tertiary referral center simulation in healthcare. The Journal of the
Society for Simulation in Healthcare 2016;11:25–31. - Eccles M, Grimshaw J, Campbell M, Ramsay C. Research designs for studies evaluating the
effectiveness of change and improvement strategies. Qual Saf Health Care 2003;12: 47–52. - IMS Institute for Healthcare Informatics. Patient adoption of mhealth, use, evidence and
remaining barriers. Parsippany (NJ): IMS Institute for Healthcare Informatics; 2015. 59p. - Mobile medical applications: guidance for industry and food drug administration staff.
February 9 2015. https://www.fda.gov/downloads/MedicalDevices/…/UCM263366.pdf
(last accessed 20170531). - ANESLIST (free checklist downloadable from Apple AppStore® and
GooglePlay® store). - Clebone A, Burian BK, Watkins SC, et al. The development and implementation of cognitive aids
for critical events in pediatric anesthesia: The Society for Pediatric Anesthesia Critical Events
Checklists. Anesth Analg 2017;124:900–907.


 Issue PDF
Issue PDF