Historical
The earliest description of curare, a naturally occurring predecessor of the neuromuscular blocking agents commonly used today in anesthesia, has been attributed to Sir Walter Raleigh in his 1596 book, The Discoverie of the Large, Rich, and Bewtiful Empyre of Guiana, in which he describes, “the most strong poyson on their arrows” used by an indigenous tribe of Guiana.1 However, Ibanez cites numerous descriptions by Spanish explorers of lethally tipped arrows used by natives of northern South America in the century preceding the publication of Raleigh’s book.2
Although Ibanez also describes therapeutic uses of what may have been curare, it was not until 1932 that West described experiments in patients with rigidity disorders at the Hospital for Epilepsy and Paralysis in Maida Vale, London; he concluded, (there was) “a definite, measurable reduction in the muscular rigidity resulting from diseases of the pyramidal and extrapyramidal motor system…[at] doses which produce no detectable signs of weakness.”3 An early therapeutic use in humans to prevent fractures occurring with convulsive therapy for depression was described by Bennett in 1940.4 The earliest description of the use of curare in general anesthesia to achieve muscle relaxation during surgery we have found was at the Homeopathic Hospital of Montreal by Griffith, published in 1942.5 In 1954, Beecher and Todd, both at Harvard and the Massachusetts General Hospital, reported their massive study of 599,548 anesthetics in 10 university hospitals in the U.S. between 1948 and 1952.6 They undertook this study because of their, “…belief that anesthesia has an unnecessarily high death rate.” All deaths were classified by a surgeon and anesthesiologist at each hospital; however, precise criteria for cause of death were not provided. A muscle relaxant was used in 2.8% (16,560), which was tubocurarine in 55%, decamethonium bromide in 37%, and succinylcholine in 4% of cases. They found 6 times as many anesthetic deaths were associated with “curare,” compared to patients managed without. Recognizing the need for risk-adjustment, 13,204 patients sampled in 1952 were classified as “good or poor physical status” (this was not the ASA classification, which had been published in 1941,7 but rather a seven-point scale devised by the authors, which was effectively similar to the ASA classification). The distribution of this scale was similar between patients receiving a NMB and those not.
Contemporary Studies
Residual NMB postoperatively has been known for more than 35 years,8 and occurs commonly despite reversal with neostigmine with a reported incidence of 4 to 50%.9,10 Studies prior to 2005, suggested residual neuromuscular block should be defined by a train-of-four ratio (TOFR) of <0.7. However, subsequent studies have discovered that residual neuromuscular blockade can occur at TOFR ≥0.9, as per the review by Murphy and Brull in 2010.11 These authors concluded that, “Residual neuromuscular block is an important patient safety issue and that neuromuscular management affects postoperative outcome.”11
Reversal of NMB
Acetylcholinesterase inhibitors, such as neostigmine, are commonly used to reverse NMB at the conclusion of surgery; however, they may have unwanted side-effects such as tachycardia, nausea, confusion, constipation, and dry mouth.12 More importantly, when used without appropriate nerve stimulator monitoring and dosing, they may actually increase NMB by creating very high concentrations of acetylcholine at the neuromuscular junction, which can have an antagonistic effect.13
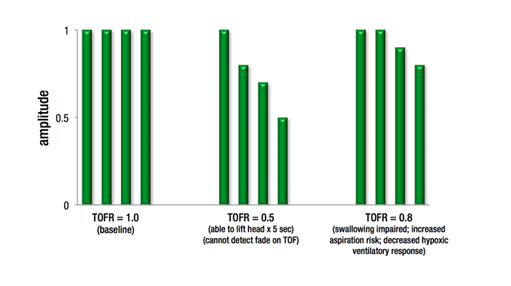
Train of four ratio (TOFR) correlation with clinical signs of reversal and ability to detect fade with TOF.
There are surprisingly few publications of adequate sample size examining the effect of NMB with and without a reversing agent on substantive outcomes important to the patient. Two of the largest studies examining this issue had significant limitations with respect to propensity matching for patient co-morbidities and/or for administration of neostigmine. These issues limit the clarity of the associations between poor outcomes and the use of NMB agents, reversal, inadequate monitoring, and inadequate reversal. These relationships are difficult to study in a retrospective manner with incomplete datasets and variable practice patterns and are better examined in large prospective studies.14,15 While some providers may believe that near complete spontaneous recovery does occur by the end of a surgical procedure without the use of NMB reversal agents, a variety of studies contradict this notion. One most notable large clinical trial by Debaene and colleagues in more than 500 patients suggested that 45% of patients examined after a single dose of an intermediate acting NMB (without a NM reversal agent) had a TOFR <0.9 in PACU.16 In addition, even 2 hours after administration of a single intermediate acting NMB, the TOFR was < 0.7 in 10% of patients and < 0.9 in 37% of the patients studied. Therefore, cautious titration of NMB reversal by using NM monitoring may reduce the risk of residual neuromuscular blockade.
Current Practice
Naguib and colleagues conducted an internet survey of active members of the Anesthesia Patient Safety Foundation and the European Society of Anaesthesiologists in 2008; 2,636 completed surveys were received.17 We did not find a more recent survey of U.S. anesthesiologists. The majority of both U.S. and European respondents estimated the incidence of clinically significant postoperative residual neuromuscular weakness to be <1%. Routine pharmacologic reversal was reported by 18% of respondents in Europe and 34% in the U.S.
Conclusions
There is a consensus in the recent literature that residual neuromuscular blockade is common and is associated with an increased risk of adverse outcomes, particularly respiratory. It is also clear that the use of NMB monitoring and appropriate reversal with neostigmine is highly variable among anesthesia providers and is thought to be primarily responsible for the high incidence of residual NMB in the recovery room.
The authors report no financial conflicts of interest related to this article and topic.
Karl E. Hammermeister, MD; Professor Emeritus, Department of Medicine (Cardiology), University of Colorado School of Medicine, Aurora CO; Surgical Outcomes and Applied Research, Department of Surgery, University of Colorado School of Medicine, Aurora CO; Adult and Child Consortium for Health Outcomes Research and Delivery Science, University of Colorado School of Medicine, Aurora CO.
Michael Bronsert, PhD; Adult and Child Consortium for Health Outcomes Research and Delivery Science, University of Colorado School of Medicine, Aurora CO; Surgical Outcomes and Applied Research, Department of Surgery, University of Colorado School of Medicine, Aurora CO.
Joshua S. Richman, MD, PhD; Department of Surgery, University of Alabama Birmingham, Birmingham VA Medical Center, Birmingham AL.
William G. Henderson, PhD; Professor, Department of Biostatistics and Informatics, Colorado School of Public Health, Aurora CO; Surgical Outcomes and Applied Research, Department of Surgery, University of Colorado School of Medicine, Aurora CO; Adult and Child Consortium for Health Outcomes Research and Delivery Science, University of Colorado School of Medicine, Aurora CO.
References
- Carman JA. History of curare. Anaesthesia 1968;23:706–707.
- Ibanez FM. Three enigmas in the history of curare before Sir Walter Raleigh. Int Rec Med Gen Pract Clin 1951; 164:700–710.
- West R. Curare in man. Proc R Soc Med 1932;25:1107–1116.
- Bennett AE. Preventing traumatic complications in convulsive shock therapy by curare. J Am Med Assoc 1940;114:322–324.
- Griffith HR. The use of curare in general anesthesia. Anesthesiol 1942;3:418–420.
- Beecher HK, Todd DP. A study of the deaths associated with anesthesia and surgery: based on a study of 599, 548 anesthesias in ten institutions 1948–1952, inclusive. Annals of Surgery 1954;140:2-35.
- Saklad M. Grading of patients for surgery. Anesthesiol 1941; 2:281–284.
- Viby-Mogensen J, Jorgensen BC, Ording H. Residual curarization in the recovery room. Anesthesiol 1979;50:539–541.
- Plaud B, Debaene B, Donati F et al. Residual paralysis after emergence from anesthesia. Anesthesiol 2010;112:1013–1022.
- Donati F. Residual paralysis: a real problem or did we invent a new disease? Canadian Journal of Anesthesiology 2013;60:714–19.
- Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg 2010;111:120–128.
- Abad-Gurumeta A, Ripolles-Melchor J, Casans-Frances R et al. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia 2015;70:1441-1452.
- Goldhill DR, Wainwright AP, Stuart CS, Flynn PJ. Neostigmine after spontaneous recovery from neuromuscular blockade. Effect on depth of blockade monitored with train-of-four and tetanic stimuli. Anaesthesia 1989;44:293–9.
- Arbous MS, Meursing AE, Van Kleef JW et al. Impact of anesthesia management characteristics on severe morbidity and mortality. Anesthesiol 2005;102:257–268.
- Grosse-Sundrup M, Henneman JP, Sandberg WS et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ 2012; 345:e6329.
- Debaene B, Plaud B, Dilly MP, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology 2003;98:1042–8.
- Naguib M, Kopman AF, Lien CA et al. A survey of current management of neuromuscular block in the United States and Europe. Anesth Analg 2010;111:110–119.


 Issue PDF
Issue PDF