The hidden universality of residual neuromuscular blockade was initially brought to the attention of anesthesiologists in 1979 by Jorgen Viby-Mogensen, who reported a 42% incidence of unidentified residual neuromuscular blockade in the recovery room (defined as a recovery of the train-of-four (TOF) ratio to 0.7).1,2 Current literature supports the idea that quantitative monitoring of the effects of neuromuscular blocking drugs reduces the likelihood of unrecognized, clinically significant residual muscle weakness in the postoperative period, which should improve patient safety.3 National anesthesia societies have taken varied stances in support of this patient safety topic. The American Association of Nurse Anesthetists focused on the neuromuscular monitoring standard as early as 1989.4 However, in 1992 when this standard was revised to ensure that monitoring of “the neuromuscular response to assess depth of blockade and degree of recovery” was included in the basic monitoring standard, the use of quantitative monitoring was not specified.5 While the American Society of Anesthesiologists (ASA) recognizes the importance of the intraoperative monitoring of neuromuscular blockade, it has not made this a component of the ASA monitoring standard. The Anesthesia Patient Safety Foundation has concluded, based on an extensive review of the literature, that residual neuromuscular blockade is a common, under-appreciated condition that contributes to adverse events in the postoperative period.6 Yet, despite the cumulative expert contributions made in this field over the past 35 years, published studies continue to report similar occurrence rates of residual neuromuscular blockade as those reported in 1979.2,7-10
Strategies to prevent residual blockade include the judicious use of neuromuscular blocking agents (NMBD), the use of quantitative neuromuscular monitoring, and the titration of reversal agents to effect.11 How then, can we explain the persistent incidence of postoperative residual neuromuscular blockade? The answer may in part be due to the over-reliance of anesthesia professionals on clinical signs, which are incapable of accurately identifying residual neuromuscular blockade. Clinical tests such as sustained head lift12-14 or grip strength15 are easy to perform, but their sensitivity to residual blockade that affects upper airway function without affecting diaphragmatic function is poor (11–14%).16 Clinicians often administer a fixed dose of reversal agent intraoperatively and are predisposed to over-interpret the clinical exam as full recovery immediately after anesthesia, when patients are barely able to participate properly. Although these clinical tests require the generation of maximum volitional muscle strength, in the presence of a fixed dose of reversal agent and a qualitative TOF twitch return, the patient’s limited test response is often attributed to anesthetic recovery rather than residual neuromuscular blockade. The suboptimal clinical test results are thus erroneously interpreted as signs of sufficient neuromuscular recovery.
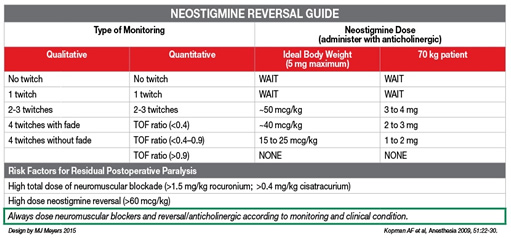
A TOF ratio greater than or equal to 0.9 indicates adequate recovery of neuromuscular transmission.17 The use of quantitative TOF monitoring in the operating room has been shown to decrease the incidence of postoperative weakness.11 The inconsistent use of objective neuromuscular transmission monitoring as well as the inappropriate dosing of neostigmine may explain the observed persistence of residual neuromuscular blockade.2,7-10 What should we do to complete Dr. Viby-Mogensen’s mission to eliminate residual neuromuscular blockade? Important next steps include interdisciplinary efforts to develop and adopt TOF-based guidelines for the intraoperative management of neuromuscular blockade and to implement these best practices consistently into clinical practice.18
Data from the Massachusetts General Hospital (MGH) revealed a significant incidence of residual neuromuscular blockade on admission to the post-anesthesia care unit (PACU).19 Furthermore, an association between inappropriate neostigmine dosing and postoperative respiratory complications was found.12 These outcomes revealed an opportunity to improve the quality of care provided with respect to intraoperative NMBD management. Under the guidance of a committee consisting of anesthesia professionals (attending anesthesiologists, CRNAs, anesthesia residents), PACU nurses, nurse practitioners, and departmental Quality Assurance (QA)/Quality Improvement (QI) leadership and personnel, a multifaceted initiative was designed to change departmental practice with respect to the intraoperative management of neuromuscular blockade. This initiative consisted of four components: implementation of an education program; distribution of a cognitive aid; provision of feedback regarding departmental progress; and the adoption of a TOF documentation requirement for our department’s quarterly QI incentive bonus. Over the course of two months, two department-wide presentations (including a case conference) were devoted to a presentation of the data regarding the incidence and effects of residual paralysis. Attending anesthesiologists, CRNAs, and anesthesia resident champions were identified and designated as informational resources for questions and concerns. Furthermore, an online repository was created with links to the relevant literature in the field. The cognitive aid, a TOF-based neostigmine-dosing guide, was developed by one of our anesthesia residents (Matthew Meyer, MD) and distributed to the members of the department in an electronic format. It was also made available to them online and affixed to each anesthesia machine (Figure 1). Our department participates in a quarterly QI bonus program. As a “nudge” towards the adoption of better NMBD management practices, we tied the quarterly QI bonus to the rate of documentation of twitches within the fifteen minutes prior to the administration of neostigmine. Our goal was to provide a reminder to evaluate neuromuscular blocking reversal dosing in a manner that was not intrusive, easy to implement, and easy to monitor. The initiative has succeeded in improving the documentation of TOF. We are currently in the process of evaluating its effects on clinical outcomes.
We know that residual neuromuscular blockade is a relevant problem that leads to a significant increase in respiratory morbidity and health care utilization.8,12,20 However, residual neuromuscular blockade remains pervasive despite the advances in our understanding of this challenge since Dr. Viby-Mogensen’s 1979 report. The fundamental issue appears to be the continued reliance by anesthesia professionals on informal and variable applications of qualitative clinical indicators rather than use of objective and quantitative TOF stimulation to determine appropriate reversal of neuromuscular blockade. The quantitative measurement of TOF stimulation is a reliable and objective measurement of adequate return of neuromuscular activity, and can be effectively used as a guide for appropriate neostigmine dosing. The QA/QI initiative at the MGH is an example of an integrated interdisciplinary approach by key stakeholders to promote sustained adoption of these best practices and improve patient safety. Broader adoption of similar evidenced-based initiatives and guidelines should provide a significant leap forward towards the elimination of the hidden universality of residual neuromuscular blockade and reduce the co-morbidities and added healthcare utilization associated with residual neuromuscular blockade.
Financial Disclosure: Dr. Eikermann has received grant funding from Merck and holds equity shares at Calabash Biotechnology. The remaining authors report no financial disclosures.
Maria van Pelt, PhD, CRNA Team Leader Nurse Anesthesia Divisions of Neurosurgery, Vascular and Thoracic Department of Anesthesia, Critical Care and Pain Medicine Massachusetts General Hospital Harvard Medical School
Hovig V. Chitilian, MD Assistant Professor of Anaesthesia Department of Anesthesia, Critical Care and Pain Medicine Massachusetts General Hospital Harvard Medical School
Matthias Eikermann, MD-PhD Associate Professor of Anaesthesia Clinical Director, Critical Care Division Department of Anesthesia, Critical Care and Pain Medicine Massachusetts General Hospital Harvard Medical School
References
- Viby-Mogensen J, Jorgensen BC, Ording H. Residual curarization in the recovery room. Anesthesiology 1979;50(6):539–541.
- Eikermann M. The hidden universality of residual neuromuscular blockade. Letter to the Editor. Available at: http://bja.oxfordjournals.org/letters/?first-index=11&hits=10&days=60. Accessed 12/31/2015.
- Brull SJ, Murphy GS. “Residual neuromuscular block: lessons unlearned. Part II: methods to reduce the risk of residual weakness.” Anesth Analg 2010;111:129–140.
- Guidelines and Standards for Nurse Anesthesia Practice: Patient Monitoring Standards. American Association of Nurse Anesthetists 1992. Available at: https://www.aana.com/newsandjournal/Documents/patient_monitoring_0492_p137.pdfAccessed 12/31/2015.
- Standards for Nurse Anesthesia Practice. American Association of Nurse Anesthetists 2013. Available at: http://www.aana.com/resources2/professionalpractice/Documents/PPM% 20Standards%20for%20Nurse%20Anesthesia%20Practice.pdf. Accessed 12/31/2015.
- Stoelting, RK. APSF survey results: Drug-induced muscle weakness in the postoperative period safety initiative. APSF Newsletter 2013-14;28:49,70-71.
- Brueckmann B, Villa-Uribe JL, Bateman BT, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology 2013;118(6):1276–1285.
- Sasaki N, Meyer MJ, Malviya SA, et al. Effects of neostigmine reversal of nondepolarizing neuromuscular blocking agents on postoperative respiratory outcomes: a prospective study. Anesthesiology 2014;121(5):959–968.
- Martinez-Ubieto J, Ortega-Lucea S, Pascual-Bellosta A, et al. Prospective study of residual neuromuscular block and postoperative respiratory complications in patients reverted with neostigmine versus sugammadex. Minerva anestesiologica 2015. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26472231. Accessed 12/31/2015.
- Murphy GS, Szokol JW, Avram MJ, et al. Residual neuromuscular block in the elderly: Incidence and clinical implications. Anesthesiology 2015;123:1322-36.
- Murphy GS, Szokol JW, Marymont JH, et al. Intraoperative acceleromyographic monitoring reduces the risk of residual neuromuscular blockade and adverse respiratory events in the postanesthesia care unit. Anesthesiology 2008;109:389–398.
- McLean DJ, Diaz-Gil D, Farhan HN, Ladha KS, Kurth T, Eikermann M. Dose-dependent association between intermediate-acting neuromuscular-blocking agents and postoperative respiratory complications. Anesthesiology 2015;122:1201–1213.
- Pavlin EG, Holle RH, Schoene RB. Recovery of airway protection compared with ventilation in humans after paralysis with curare. Anesthesiology 1989;70:381-385.
- Dam WH, Guldmann N. Inadequate postanesthetic ventilation. Curare, anesthetic, narcotic, diffusion hypoxia. Anesthesiology 1961;22:699-707.
- Bodman, RI. Evaluation of two synthetic curarizing agents in conscious volunteers. Br J Pharmacol Chemother 1952;7:409-416.
- Debaene B, Plaud B, Dilly M-P, Donati, F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology 2003;98:1042-1048.
- Murphy GS, Szokol JW. Monitoring neuromuscular blockade. Int Anesthesiol Clin 2004;42(2):25–40.
- Brull SJ, Prielipp RC. Reversal of neuromuscular blockade: “identification friend or foe.” Anesthesiology 2015;122:1183–1185.
- Brueckmann B, Sasaki N, Grobara P, et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: a randomized controlled study. Br Anaesth 2015;115:743–751.
- Staehr-Rye AK. GS, Grabitz SD, Thevathasan T, Sasaki N, Meyer MJ, Gätke MG, Eikermann E. Effects of residual paralysis on postoperative pulmonary function and hospital length of stay. ASA Abstract 2015; A4026.


 Issue PDF
Issue PDF