![]() Dear Q&A,
Dear Q&A,
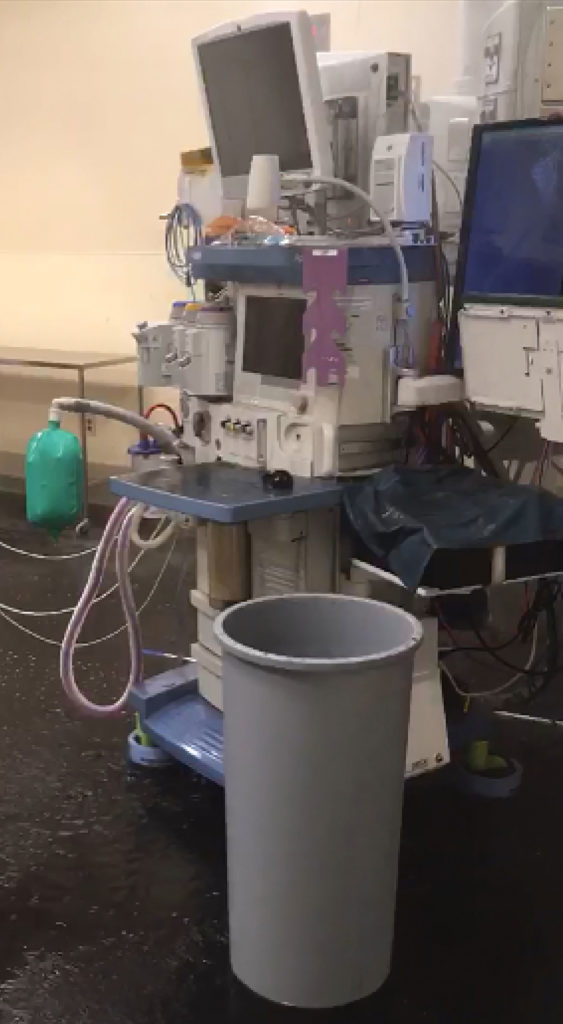
A recent event in our operating room has led me to inquire about the suitability and/or safety of using an anesthesia machine that has been saturated with water due to a ceiling sprinkler activation. A sprinkler head in one of our operating rooms was inadvertently activated. The anesthesia machine (Dräger Apollo-2012, Telford, PA) was directly below the sprinkler, and as a result, was saturated with water (Figures 1 & 2). After the machine had dried, the inspector tested the machine, and it has been deemed clear to use. I am told no infection control tests were performed, only a test of machine functionality.
The affected machine was removed from service at the time of the event and has remained out of service. My question is whether this machine is safe for patient use from an infection control perspective moving forward. There was standing water in and on this machine during the sprinkler activation. Are there other standards or tests we should be adhering to before putting this machine back into service?
Thank you for your time,
Brooke L. Williams, MNA, CRNA, APNP
Milo C. Huempfner VA Clinic
Green Bay, WI
This author has no conflicts of interest to report.
![]() Dear Ms. Williams,
Dear Ms. Williams,
Dräger would like to thank the Anesthesia Patient Safety Foundation (APSF) for the opportunity to respond to the above submission.
The author describes a situation where an Apollo anesthesia machine was beneath an operating room sprinkler head, and the sprinkler was inadvertently activated resulting in an Apollo anesthesia machine saturated with water. The author is asking if the Apollo can be put back into service, and if so, are there any concerns from an infection control standpoint.The Apollo anesthesia machine Instructions For Use (IFU) specifications state that the Apollo must be stored in an environment of 25% to 85% relative humidity (no condensation). In the case of the activated sprinkler head, this specification was violated. Although the author states that the machine checked out functionally, Dräger cannot guarantee there is not internal contamination or other related conditions resulting from the exposure to water that could lead to future device malfunction. Corrosion and/or mold/mildew may also be a future concern. It is Dräger’s recommendation that the machine should not be used until the potential internal contamination and/or latent damage is assessed and resolved, if possible, by an expert in this field. This type of assessment is beyond the expertise of Dräger. If this type of assessment is not possible, it is Dräger’s recommendation that the machine be replaced and not be put back into use.
In summary, Dräger would like to thank the authors for sharing this unique scenario with the anesthesia community.
Thank you,
David Karchner
David Karchner is senior director of Marketing, Operating Room, Service, and Government Solutions at Dräger Medical.
![]() Dear Ms. Williams,
Dear Ms. Williams,
The APSF Newsletter receives interesting letters that often challenge our knowledge and expertise.
Water saturation of an anesthesia machine from a ceiling sprinkler activation is an exceedingly rare event, and it is difficult to provide a response that is well supported by data or past experience. The response from Dräger Medical, the manufacturer, is very useful as it underscores the challenge to finding truly expert advice on the best course of action. This experience raises the more general question about the role of the anesthesia professional in assessing the safety of equipment to be used for patient care.
Ms. Williams is to be commended for questioning the safety of this anesthesia machine after such significant water exposure. Although the hospital biomedical technicians can be helpful in this situation, the ultimate responsibility lies with the anesthesia professional to be as certain as possible that equipment is safe before beginning an anesthetic. One can imagine the pressure on a hospital administrator to keep this expensive device in clinical use maintaining continued use of the operating room and avoiding the cost of replacement. The anesthesia professional is uniquely qualified to raise concerns about the safety of the equipment that administration may not appreciate.
The report mentions that an “inspector” deemed the machine clear to use. It is not clear from this report how the inspector was qualified to render an opinion. Technicians are not required to be manufacturer certified to provide service to anesthesia machines and many are not. Even technicians who are manufacturer certified can be independent contractors and may not have access to the manufacturer for an opinion. In this case, an official opinion from the manufacturer is warranted and although it may not be the desired opinion, it does clarify the potential liability of continuing to use the machine.
Even though an inspector indicated the machine is clear to use, given the information from the manufacturer, it is not clear who might be able to provide a sufficient expert opinion to warrant continued use of the device. If the machine did stay in service and resulted in patient injury, not only would the caregivers be burdened by their sense of responsibility for the patient’s injury, but the liability could be difficult to defend.
Practicing with a questioning attitude is a core patient safety principle. Whenever there is a concern about the safety of medical devices for patient care, the anesthesia professional should not begin an anesthetic until the concern has been addressed. If an inspection is performed, the qualifications of the certifying inspector should be documented, and, if necessary, an opinion from the manufacturer should be sought.
Dr. Feldman is chair, APSF Committee on Technology, and professor of Clinical Anesthesiology, Children’s Hospital of Philadelphia Perelman School of Medicine, Philadelphia, PA.
Dr. Feldman has received consulting compensation from Micropore, Dräger Medical, GE Medical, and Medtronic.
The APSF sometimes receives questions that are not suitable for the Rapid Response column. This Q and A column allows the APSF to forward these questions to knowledgeable committee members or designated consultants. The information provided is for safety-related educational purposes only, and does not constitute medical or legal advice. Individual or group responses are only commentary, provided for purposes of education or discussion, and are neither statements of advice nor the opinions of the APSF. It is not the intention of the APSF to provide specific medical or legal advice or to endorse any specific views or recommendations in response to the inquiries posted. In no event shall the APSF be responsible or liable, directly or indirectly, for any damage or loss caused or alleged to be caused by or in connection with the reliance on any such information.


 Issue PDF
Issue PDF