Recent reports about unrecognized cerebral ischemia under general anesthesia are alarming,1,2 and reveal that it may start insidiously, progress covertly, and result in devastating outcomes. Although much of the emphasis for anesthesia management is focused on maintenance of adequate cerebral perfusion pressure (CPP), does this ensure sufficient perfusion of the brain? Perfusion pressure by itself is the propulsion force only; it does not determine distribution of cerebral blood flow (CBF), ensure adequate collateral circulation, nor account for variations in the venous outflow path. Here we present a case that demonstrates the utility as well as limitations of processed EEG monitoring to assess adequacy of the CBF during an episode of postural cerebral venous steal.
To measure CPP, an arterial catheter is placed and zeroed at the level of the external acoustic meatus to approximate the circle of Willis. Most anesthesiologists and nurse anesthetists aim to maintain CPP within 15-20% of the baseline value. To calculate CPP in the sitting position one needs to know both arterial (inflow) and venous (effective outflow) pressures.3 We can easily measure the arterial (inflow) pressure, but we usually only estimate the effective outflow pressure. When outflow through the vertebral venous plexus is not adequate, atmospheric pressure assumes the role of effective backpressure due to jugular vein compression at the skull base.3,4 In this context pressure measurement at the external acoustic meatus estimates the CPP, assuming that the effective outflow pressure is zero (atmospheric) at the scull base.3
We also can only assume that the effective outflow pressure is uniform throughout the brain. Regional differences in the effective outflow pressure in the brain can cause a cerebral venous steal phenomenon diverting blood flow to the pathway of least resistance.5 During head-up tilt, atmospheric pressure hinders outflow from the upper body with zero venous pressure and diverts the flow to the lower body where venous pressure exceeds atmospheric (postural “steal”). Similarly, alveolar pressure diverts pulmonary blood flow into dependent parts of pulmonary circulation.6,7 Thus the adequacy of cerebral perfusion can not be determined solely from the arterial pressure, but requires consideration of the effects of vascular anatomy, autoregulation, PaCO2, anesthetic, viscosity, vascular tone, and regional variation of these factors on the CBF. More direct assessment of cerebral perfusion would be desirable.
Ideally, a sensitive neurological exam can assess potential compromise of cerebral function occurring during periods of inadequate cerebral perfusion. However, under general anesthesia assessment of cerebral function is more challenging. Although many options are available, no single monitoring modality has been demonstrated as superior.8
Neurophysiologic monitoring with evoked potentials requires dedicated trained staff and equipment and is typically limited to select cases. Transcranial Doppler monitoring of middle cerebral artery flow velocity is operator-dependent, very sensitive to the acoustic contact, and fails when the acoustic “window” cannot be found. Jugular vein oximetry is invasive. Near infrared oximetry monitors tissue oxygenation in hair-free areas of the head but is not broadly available. In contrast, processed EEG monitoring devices are widespread, and although typically utilized to help manage intraoperative anesthetic dosing, they could help to detect cerebral hypoperfusion.
Case Report
A 34-year-old female, 5’8″, 76.2 kg underwent laparoscopic cholecystectomy under general endotracheal anesthesia. Medical history was significant for hypertension, controlled with metoprolol. She had previously undergone gastric bypass surgery for morbid obesity. Following induction, anesthesia was maintained with sevoflurane 2% inspired in air/oxygen. The patient’s baseline blood pressure was 143/97 mmHg; the immediate preinduction value was 167/113 mmHg; and following induction was 130/80 mmHg. Approximately 40 min after induction, with pneumoperitoneum and reverse Trendelenburg position, blood pressure was noted to decrease acutely to 95/50. At this point, a BIS monitor was applied and the initial BIS value was 20 with sevoflurane end-tidal value at 1 MAC. Two doses of ephedrine (5 mg + 10 mg) were administered. Blood pressure promptly returned to 130/80 and BIS simultaneousely increased to 40-50 without decreasing the MAC (see Figure 1). The case was completed and the patient recovered uneventfully.
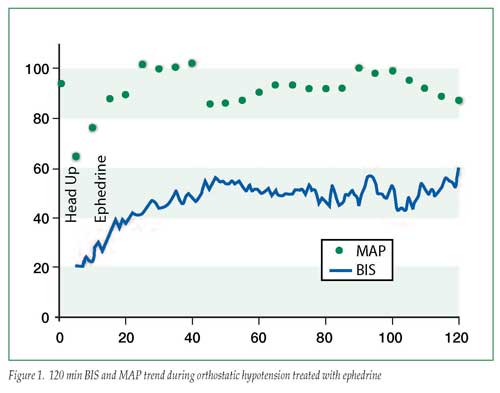
We submitted the BIS trend data for review by the manufacturer (Aspect Medical Systems) who confirmed that BIS was functioning as designed. Interrogation of the trend recording revealed the BIS system was detecting delta wave activity at the beginning of the record and the BIS values were appropriately low during the initial readings.
Discussion
Although the BIS Index was originally developed to measure the effects of anesthetic and sedative agents, multiple case reports have described the ability of BIS monitoring to detect episodes of cerebral ischemia.9-17 In the absence of larger validating studies, sensitivity and specificity of BIS for this application has been questioned.13,18,19 Although we believe our case demonstrates the ability of processed EEG monitoring to detect cerebral hypoperfusion, it also highlights important limitations. Without a baseline record prior to and following induction of anesthesia, a low BIS value during orthostatic hypotension may reflect the effect of the anesthetic on the EEG with or without the additive effects of hypoperfusion. In our patient, we observed rapid resolution of the low BIS values following ephedrine administration. Although ephedrine increases blood pressure and CBF, it has also been reported to increase BIS values directly.20 Similar to cerebral oximetry, BIS and other processed EEG monitors analyze EEG signals from the frontal lobe only, and consequently may miss regional CBF abnormalities. Although our patient exhibited low BIS values during the period of postural hypotension, it should be noted that a variety of artifact conditions may cause spuriously elevated BIS values.13
Conclusion
Processed EEG monitoring systems are operator- independent, widely available, relatively inexpensive technologies to use during the intraoperative period. Although these technologies are not specifically developed as monitors for cerebral perfusion, they may help detect otherwise unrecognized global cerebral ischemia. If anesthetic dosing and surgical stimulation are stable, and postural changes or acute hypotension result in a precipitous decrease in the brain function values, clinicians may consider correcting the hypotension till brain function values improve.
The recent case reports should remind us that the brain is a critical and fragile organ that we rarely monitor directly during general anesthesia. We do not believe that the absence of the “perfect” cerebral monitor should discourage us from obtaining the most information from available modalities. We believe that available processed EEG monitoring devices could help in this regard. Larger scale validation studies to determine processed EEG and CBF correlates could advance functional cerebral monitoring and improve patient safety.
The authors acknowledge and appreciate the technical help from Aspect Medical Systems, Inc. for the data extraction from the BIS monitor, and the editorial review and comments from Dr. Scott Kelley.
Disclosure: Drs. Mindaugas and Osvaldas have no financial relationship with Aspect Medical Systems, Inc. Aspect Medical Systems is a corporate donor to the APSF.
Dr. Mindaugas Pranevicius is an assistant professor at the Albert Einstein College of Medicine, Jacobi Medical Center, Bronx, NY, and Dr. Osvaldas Pranevicius is an attending anesthesiologist at the New York Hospital Queens, Flushing, NY.
- Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth 2005;17:463-9.
- Cullen DJ, Kirby RR. Beach chair position may decrease cerebral perfusion; catastrophic outcomes have occurred. APSF Newsletter 2007;22(2):25-7.
- Pranevicius M, Pranevicius O. Modified calculation of the cerebral perfusion pressure in a sitting position: jugular Starling resistor and related clinical implications. APSF Newsletter. 2008;23(2):32-3.
- Valdueza JM, von Münster T, Hoffman O, et al. Postural dependency of the cerebral venous outflow. Lancet 2000;355:200-1.
- Pranevicius M, Pranevicius O. Cerebral venous steal: blood flow diversion with increased tissue pressure. Neurosurgery 2002;51:1267-74.
- West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lung: relation to vascular and alveolar pressures. J Appl Physiol 1964;19:713-24.
- Lopez-Muniz R, Stephens NL, Bromberger-Barnea B, et al. Critical closure of pulmonary vessels analyzed in terms of Starling resistor model. J Appl Physiol 1968;24:625-35.
- Moritz S, Kasprzak P, Arlt M, et al. Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: a comparison of transcranial Doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology 2007;107:563-9.
- Adam N, Sebel PS. BIS monitoring: awareness and catastrophic events. Semin Cardiothorac Vasc Anesth 2004;8:9-12.
- Bonhomme V, Desiron Q, Lemineur T, et al. Bispectral index profile during carotid cross clamping. J Neurosurg Anesthesiol 2007;19:49-55.
- el-Dawlatly AA. EEG bispectral index during carotid endarterectomy. Middle East J Anesthesiol 2003;17:287-93.
- Mérat S, Lévecque JP, Le Gulluche Y, et al. BIS monitoring may allow the detection of severe cerebral ischemia. Can J Anaesth 2001;48:1066-9.
- Myles PS, Cairo S. Artifact in the bispectral index in a patient with severe ischemic brain injury. Anesth Analg 2004;98:706-7.
- Umegaki N, Hirota K, Kitayama M, et al. A marked decrease in bispectral index with elevation of suppression ratio by cervical haematoma reducing cerebral perfusion pressure. J Clin Neurosci 2003;10:694-6.
- Azim N, Wang CY. The use of bispectral index during a cardiopulmonary arrest: a potential predictor of cerebral perfusion. Anaesthesia 2004;59:610-2.
- Morimoto Y, Monden Y, Ohtake K, et al. The detection of cerebral hypoperfusion with bispectral index monitoring during general anesthesia. Anesth Analg 2005;100:158-61.
- Prabhakar H, Ali Z, Rath GP, Singh D. Zero bispectral index during coil embolization of an intracranial aneurysm. Anesth Analg 2007;105:887-8.
- Dahaba AA. Different conditions that could result in the bispectral index indicating an incorrect hypnotic state. Anesth Analg 2005;101:765-73.
- Deogaonkar A, Vivar R, Bullock RE, et al. Bispectral index monitoring may not reliably indicate cerebral ischaemia during awake carotid endarterectomy. Br J Anaesth 2005;94:800-4.
- Ishiyama T, Oguchi T, Iijima T, et al. Ephedrine, but not phenylephrine, increases bispectral index values during combined general and epidural anesthesia. Anesth Analg 2003;97:780-4.


 Issue PDF
Issue PDF