Episode #252 Managing Neurologic Stimulators: A Critical Guide for Safe Anesthesia
April 30, 2025Welcome to the next installment of the Anesthesia Patient Safety podcast hosted by Alli Bechtel. This podcast will be an exciting journey towards improved anesthesia patient safety.
Happy #250 episodes of the Anesthesia Patient Safety Podcast!!
We are returning to the February 2025 APSF Newsletter article today. Our featured article is “Recommendations for Managing Non-Cardiac Implantable Electrical Devices (NCIEDs) During Non-Neurologic Surgery and Procedures” by Jacqueline Morano and Jamie Uejima.
Today, we are talking about non-cardiac implantable electrical devices or neurologic stimulators and focusing on the preoperative period.
Check out Figure 1 in the article for important considerations:
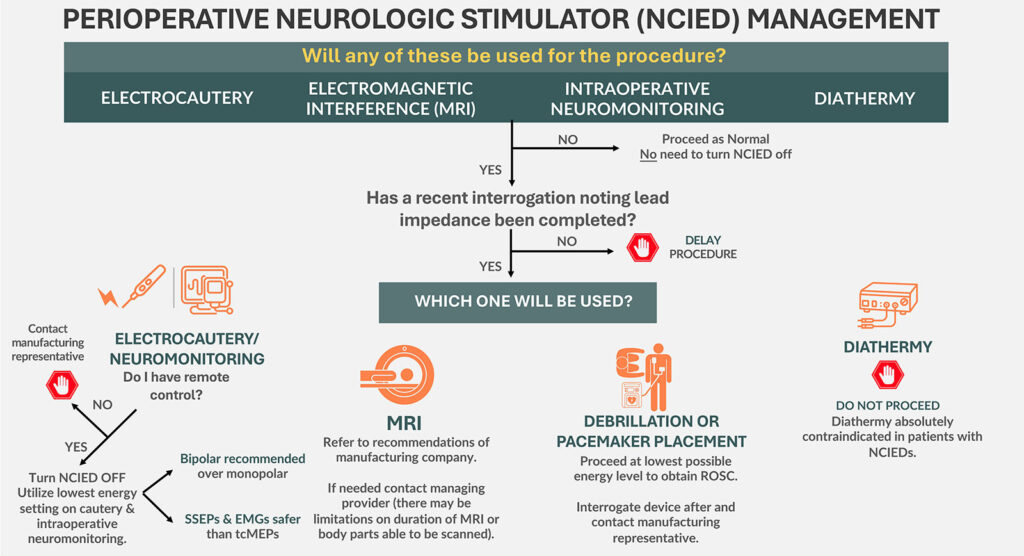
Figure 1: Potential intraoperative device interactions when patients present with NCIEDs.
NCIED: Non-cardiac implantable electrical device; MRI: Magnetic Resonance Imaging; SSEP: Somatosensory Evoked Potential; EMG: Electromyography; tcMEPs: Transcortical Motor Evoked Potential; ROSC: Return of Spontaneous Ventilation
Check out Table 1 in the article for everything you need to conduct a complete preoperative evaluation for a patient with an NCIED.
Table 1: General Perioperative Concerns for Patients with NCIEDs2
| Identify the type of device, the manufacturer, and model. Does the patient have a device identification card? |
| Where are the leads and pulse generator located? |
| How is the device turned off or inactivated? Does the patient have a remote or magnet? |
| What symptoms develop when the device is turned off? |
| When was the device implanted? What is the battery status? |
| When was the last device check/interrogation? |
| What was the lead impedance on the last device check? |
| Determine availability of “safe modes” for surgery or for MRI. |
| The provider who placed the device should be contacted for perioperative device recommendations (as part of preoperative clinic assessment). |
| Does the surgery/procedure require neuromonitoring? If so, discuss with the provider who placed the device as certain neuromonitoring modalities may be deemed unsafe (preop. clinic provider). |
| Contact the device representative to determine if they need to be present on the day of surgery for pre/postoperative interrogation (preop. clinic provider). |
| Does the patient also have any additional implanted devices? If so, the providers managing both devices should be contacted for recommendations. |
We also discuss an urgent Patient Safety Alert about Medication Vial Coring and Fragmentation Risks that was published on APSF.org on March 30, 2025. You can check it out here. https://www.apsf.org/news-updates/patient-safety-alert-urgent-alert-regarding-medication-vial-coring-and-fragmentation-risks/
Here are the interim Practice Recommendations to Minimize the Risk of Coring When using Needles to Access Medication Vials include the following:
- Consult and follow the manufacturer’s package insert of the intended product for specific recommendations on accessing the vial. If instructions are not provided, avoid the use of blunt needles. Use sharp needles, ideally with needle guard protection.1
- Smaller gauge needles are preferred. Consider using 21-gauge rather than 18-gauge needles when possible.2
- The angle at which the vial is entered may help to reduce the risk but recommendations for the optimal angle (45-60 degrees) are inconsistent and not supported by objective data.3,4 When using a sharp needle, an angle that creates the least resistance to puncture seems desirable.
- Puncture the vial stopper only one time.2
- Inspect the vial for macroscopic coring and if present:
-
- Do not administer the medication to the patient if coring is suspected or visible.
- Secure the affected vial and drawn up medication.1
- Contact pharmacy for assistance with reporting and returning affected vials to the manufacturer.
-
- Report any instances of coring directly to ISMP at this link: https://home.ecri.org/pages/ecri-ismp-error-reporting-system. Useful information to include in the report includes the medication, manufacturer, vial lot number, needle type (sharp or blunt) and gauge used to access the vial. Photos of the visual evidence of coring are also helpful.
This episode was edited and produced by Mike Chan.
Subscribe to our YouTube Channel here: https://www.youtube.com/@AnesthesiaPatientSafety
Be sure to check out the APSF website at https://www.apsf.org/
Make sure that you subscribe to our newsletter at https://www.apsf.org/subscribe/
Follow us on Twitter @APSForg
Questions or Comments? Email me at [email protected].
Thank you to our individual supports https://www.apsf.org/product/donation-individual/
Be a part of our first crowdfunding campaign https://www.apsf.org/product/crowdfunding-donation/
Thank you to our corporate supporters https://www.apsf.org/donate/corporate-and-community-donors/
Additional sound effects from: Zapsplat.
© 2025, The Anesthesia Patient Safety Foundation
Hello and welcome back to the Anesthesia Patient Safety Podcast. My name is Alli Bechtel, and I am your host. Thank you for joining us for another show. We just celebrated a major birthday milestone for the Anesthesia Patient Safety Podcast. Did you know that there are over 250 episodes!! If you are just joining us today for the first time or you have been listening every week, we are so glad that you are here and making the commitment to work towards improved anesthesia patient safety. Now, quick go tell a friend or colleague about our show. We would love to reach even more people on our way to 500 episodes. You can tell them that today’s episode is all about unraveling the mysteries of non-cardiac implantable electrical devices and how to keep your patients with these devices safe.
Before we dive further into the episode today, we’d like to recognize Vertex, a major corporate supporter of APSF. Vertex has generously provided unrestricted support to further our vision that “no one shall be harmed by anesthesia care”. Thank you, Vertex – we wouldn’t be able to do all that we do without you!”
We are returning to the February 2025 APSF Newsletter article today. Our featured article is “Recommendations for Managing Non-Cardiac Implantable Electrical Devices (NCIEDs) During Non-Neurologic Surgery and Procedures” by Jacqueline Morano and Jamie Uejima. To follow along with us, head over to APSF.org and click on the Newsletter heading. First one down is the Current Issue. Then, scroll down until you get to our featured article today. I will include the link in the show notes as well.
To help kick off the show today, we are going to hear from one of the authors to give us the inside scoop.
Here she is now.
[Morano] “Hello, my name is Jacqueline Morano, and I am an anesthesiologist at Northwestern Memorial Hospital in Chicago, Illinois, and the first author of recommendations for managing non-cardiac implanted electrical devices during non-neurologic surgery and procedures published in February 2025 edition of the APSF newsletter.”
[Bechtel] I asked Morano why she wrote this article. Let’s take a listen to what she had to say.
[Morano] “We wrote this article as at our own institution, we have encountered several cases where our colleagues are unsure of what to do when they have one of these devices in their patients with so little in the literature. We thought we would provide a more comprehensive approach for anesthesia providers and provide some guidance as to what to do when they too have a patient with a non-cardiac implanted electrical device.”
[Bechtel] Thank you so much to Morano for introducing this topic. Have you ever provided anesthesia care for a patient with a vagal nerve stimulator, a deep brain stimulator, or a spinal cord stimulator? How did you manage the device? We are so excited to get into this article and talk about how to keep patients with these devices safe during anesthesia care. Here we go.
Today, we are talking about non-cardiac implantable electrical devices or neurologic stimulators. The most common devices are spinal cord stimulators, deep brain stimulators, and vagal nerve stimulators. Another example is the Hypoglossal Nerve Stimulator that is used to help treat obstructive sleep apnea by sending electrical pulses to the hypoglossal nerve which controls tongue movement and causes the tongue to move forward during sleep to reduce airway collapse and possible obstruction.
Other devices include the:
- Phrenic Nerve Stimulator
- Sacral Nerve Stimulator
- Gastric Nerve Stimulator
The indications for these devices have increased over time so it is likely that you will provide anesthesia care for a patient with one of these devices for elective or emergent surgical procedures.
Let’s take a closer look at the most common devices. First up, we have vagal nerve stimulators. These are pulse generators that are positioned in the mid-cervical neck, usually on the left. The position is important because right-sided vagal nerve stimulation may cause severe bradycardia. Indications for this device include seizure reduction, cluster headache prevention, and refractory depression.
Next, we have deep brain stimulators. These involve an implanted lead to stimulate, well, deep brain structures including the thalamus, globus palladium, and subthalamic nuclei. The target structure depends on the indication for treatment. This is considered a minimally invasive targeted neurosurgical intervention that may be used to treat Parkinson’s disease as well as other movement disorders including tremors, tics, and dystonias, psychiatric illnesses including major depression and obsessive-compulsive disorder, chronic pain, and refractory epilepsy.
Spinal Cord Stimulators are used to inhibit chronic pain by continuously stimulating the large diameter afferent fibers in the spinal cord. The electrode is positioned in the dorsal epidural space at the level depending on the pain being treated. Low thoracic to lumbar placement is used to manage lower extremity pain and chronic low back pain and mid-cervical to high thoracic placement is used to manage upper extremity pain.
Now, let’s head to the preoperative clinic to meet our first patient who has, you guessed it, a non-cardiac implantable electrical device. It is important to perform a preoperative evaluation in the anesthesia preoperative clinic prior to elective procedures to help identify patients with these devices, contact the clinician managing the device, and inform the anesthesia professionals who will be providing care for the patient. Check out Figure 1 in the article and we are going to review the potential intraoperative device interactions now. I will include this figure in the show notes as well. The first question is “Will any of these be used for the procedure, electrocautery, electromagnetic interference or MRI, intraoperative neuromonitoring, or diathermy? If the answer to this question is No, then you may proceed as normal. You do not need to turn off the device or make any changes.
If the answer is YES, then we need to know if a recent device interrogation has been completed with information about lead impedance. If the interrogation has not been completed, then the elective procedure should be delayed until this is done.
Once you have a recent interrogation, the next step depends on what will be used during the procedure.
If electrocautery or neuromonitoring will be used, you need to determine if you have remote control of the device. If remote control is unavailable, then the next step is to contact the manufacturing representative. If remote control is available, then you will need to turn off the device and use the lowest energy setting for the electrocautery or neuromonitoring. Bipolar is recommended over monopolar. SSEPs and EMGs are safer than MEPs for neuromonitoring.
If our patient needs an MRI, then it is important to refer to the recommendations from the manufacturing company. You may need to contact the managing clinician to determine if there are any limits for the length of time for the MRI or what body parts may be scanned.
If diathermy will be used during the procedure, do NOT proceed. This is an absolute contraindication. I’ll repeat, diathermy is absolutely contraindicated for patients with non-cardiac implantable electrical devices.
Now, we are not able to plan for this one, but what if our patient requires defibrillation or pacemaker placement? It is important to proceed with the lowest possible energy level to obtain return of spontaneous circulation. Then, you will need to interrogate the device after the event and contact the manufacturing representative.
The authors also provide us with a table of important considerations for patients with these devices. These are definitely patients that you want to see in the preoperative clinic so that you have time to address these questions and make a plan for the day of the surgery. I will include Table 1 in the show notes as well. You may want to include Figure 1 and Table 1 in your preoperative clinic information folders so that you are prepared the next time you evaluate a patient with one of these devices. Let’s review the general perioperative concerns now.
- Identify the type of device, the manufacturer, and model. Does the patient have a device identification card?
- Where are the leads and pulse generator located?
- How is the device turned off or inactivated? Does the patient have a remote or magnet?
- What symptoms develop when the device is turned off?
- When was the device implanted? What is the battery status?
- When was the last device check/interrogation?
- What was the lead impedance on the last device check?
- Determine availability of “safe modes” for surgery or for MRI.
- The provider who placed the device should be contacted for perioperative device recommendations (as part of preoperative clinic assessment).
- Does the surgery/procedure require neuromonitoring? If so, discuss with the provider who placed the device as certain neuromonitoring modalities may be deemed unsafe (preop. clinic provider).
- Contact the device representative to determine if they need to be present on the day of surgery for pre/postoperative interrogation (preop. clinic provider).
- Does the patient also have any additional implanted devices? If so, the providers managing both devices should be contacted for recommendations.
The recent device interrogation with lead impedance is critical information to determine the electrical performance and structural integrity of the leads in the device. If there are changes in the lead impedance, the procedure may need to be delayed. It is also important to communicate with the surgeon so that they are aware that the patient has a device and determine if there are any special surgical needs that will be used on the day of the surgery such as electrocautery or neuromonitoring. Then, you can work through the algorithm in Figure 1 to determine if those needs will interact with the device and if the device needs to be reprogrammed to a specific setting such as MRI safe mode or surgery safe mode or if the device needs to be turned off.
Well, we made it through the preoperative time period, but you will have to tune in next week when we bring our patient with a non-cardiac implantable electrical device to the operating theatre. We’ll be talking about intraoperative and postoperative considerations including regional anesthesia options and more. So, stay tuned.
We have an urgent Patient Safety Alert to discuss about Medication Vial Coring and Fragmentation Risks that was published on APSF.org on March 30, 2025. To follow along with us, head over to APSF.org and click on the Patient Safety Resources heading. The 6th one down is News and Updates, and you will see this alert. The alert was issued by the Anesthesia Patient Safety Foundation and the ECRI and Institute for Safe Medication Practices. This is in response to several recent reports of vial coring incidents. Coring occurs when a piece of the flexible stopper on a medication vial detaches during needle insertion which may lead to contaminating the medication and the risk of injecting stopper fragments into patients. Coring may occur with any needle used to access a flexible vial stopper, but the highest risk appears to be with blunt needles. This is not a new problem, but the data supporting best practices for accessing vials remains limited. Given the recent surge in reports, the APSF and ECRI/ISMP advise that there is a real potential risk to patients and careful consideration of practices for accessing medication to minimize the occurrence of coring is required.
Work is being undertaken now to develop well-documented, evidence-based recommendations. AT this time, we have interim recommendations based on the current available data. The interim Practice Recommendations to Minimize the Risk of Coring When using Needles to Access Medication Vials include the following:
- Consult and follow the manufacturer’s package insert of the intended product for specific recommendations on accessing the vial. If instructions are not provided, avoid the use of blunt needles. Use sharp needles, ideally with needle guard protection.1
- Smaller gauge needles are preferred. Consider using 21-gauge rather than 18-gauge needles when possible.2
- The angle at which the vial is entered may help to reduce the risk but recommendations for the optimal angle (45-60 degrees) are inconsistent and not supported by objective data.3,4 When using a sharp needle, an angle that creates the least resistance to puncture seems desirable.
- Puncture the vial stopper only one time.2
- Inspect the vial for macroscopic coring and if present:
- Do not administer the medication to the patient if coring is suspected or visible.
- Secure the affected vial and drawn up medication.1
- Contact pharmacy for assistance with reporting and returning affected vials to the manufacturer.
- Report any instances of coring directly to ISMP by clicking on the link in the show notes. Useful information to include in the report includes the medication, manufacturer, vial lot number, needle type (sharp or blunt) and gauge used to access the vial. Photos of the visual evidence of coring are also helpful.
Remember, there are no documented cases of patient harm at this time, but this is a threat to patient safety if these fragments are inadvertently injected into patients. These recommendations only apply to the use of needles for accessing medication vials with flexible stoppers. The APSF and ECRI/ISMP do not offer any guidance on the use of alternate transfer devices. More work is being done in this area, so stay tuned for more information, results, and any updates to the guidelines.
If you have any questions or comments from today’s show, please email us at [email protected]. Please keep in mind that the information in this show is provided for informational purposes only and does not constitute medical or legal advice. We hope that you will visit APSF.org for detailed information and check out the show notes for links to all the topics we discussed today.
Thanks for listening. If you enjoy listening to the Anesthesia Patient Safety Podcast, and we hope that you do, please take a minute to give us a 5-star rating, subscribe, and share this podcast with your colleagues and anyone you know who is interested in anesthesia patient safety.
Until next time, stay vigilant so that no one shall be harmed by anesthesia care.
© 2025, The Anesthesia Patient Safety Foundation

