As perioperative physicians, we are largely concerned with postoperative respiratory compromise because of its impact on morbidity and mortality, as well as on healthcare costs. Respiratory compromise following surgery and/or sedation is an umbrella definition that encompasses causes of both hypoxia and hypoventilation. Without intervention, respiratory compromise can lead to a variety of complications including pneumonia, reintubation and respiratory arrest. Such complications can be attributed to the type of surgery, anesthesia, and/or patient risk factors. Some studies have found that up to 14.2% of all surgical patients experience postoperative pulmonary complications, particularly those with open upper abdominal procedures.1 It is widely believed that the induction and maintenance of anesthesia may be a contributing factor to the development of postoperative pulmonary complications due to the “disruption of the normal activity of the respiratory muscles,”2 ultimately leading to atelectasis and hypoxia.
According to Zhan et al., postoperative respiratory failure (not including pulmonary embolism) added approximately 9 hospital days to hospital length of stay, greater than $53,000 to hospital costs, and an almost 22% increase in mortality.3 It is thus evident that postoperative respiratory complications have significant and widespread sequelae for both the patient and the health care system.
Several independent risk factors for postoperative pulmonary failure have been identified. In a greater than 80,000 subject study, Arozullah et al. found that 3.4% of patients undergoing noncardiac surgery suffered postoperative pulmonary failure. Respiratory failure was defined as “mechanical ventilation for more than 48 hours after surgery or the need for reintubation after postoperative extubation.”4 Examples of such risk factors are hypoalbuminemia, advanced age (>70 years old), renal insufficiency, type of surgery (i.e., AAA, thoracic), emergency surgery, general anesthesia, COPD, and dependency status.4
Numerous risk factors for postoperative opioid-induced respiratory depression have been identified including older age, very young age, obesity, obstructive sleep apnea, neurologic disease, cardiovascular disease, and others.4,5 In fact, so many risk factors have been identified that many experts believe that all patients receiving opioids postoperatively should be monitored for respiratory depression. This argument to monitor all patients postoperatively is further strengthened when one considers all the risk factors for postoperative pulmonary complications that are not related to opioid administration.5,6
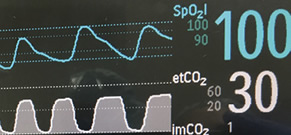
Figure 1. Pulse oximetry and capnography waveforms.
With the evolution of technology, noninvasive measures of end-tidal carbon dioxide are now available in the perioperative setting. In addition to mainstream sampling, which detects carbon dioxide levels at the endotracheal tube, side stream sampling via a cannula-like device can be used for both intubated and non-intubated patients. A numerical value and graph are displayed (Figure 1). The waveform itself can also be used as a diagnostic tool with minimum training. For example, an up sloping graph may indicate acute bronchospasm.
An increasing amount of evidence supports the use of capnograpy for earlier and more reliable warnings of respiratory depression for procedural sedation.7 The argument made in favor of capnography often cites that changes in capnography will precede changes in pulse oximetry. In a study by Burton et al., emergency department physicians administering procedural sedation were blinded to the use of capnography, as it was not the standard of care. Thirty-three percent of the cases had an adverse respiratory event (defined as a change in etCO2 of 10mmHg or greater). Of these, 70% were detected by capnography 12 to 271 seconds before changes in pulse oximetry or respiratory rate.8
It is common practice to monitor patients in the acute postoperative period with pulse oximetry and respiratory rate. This, however, may not be adequate. Many have suggested that supplemental oxygen and the presence of the PACU nurse may be potential confounders in the accurate assessment of a patient’s respiratory status. A patient may maintain his/her oxygen saturation for quite some time with supplemental oxygen despite inadequate ventilation. Moreover, hypoventilation from excessive sedation with or without upper airway obstruction may be masked by periodic stimulation by the nurse. When the nurse walks away, bradypnea, poor inspiratory effort, and/or upper airway obstruction once again ensues, leading to hypercarbia and subsequent increasing somnolence. Thus, although current guidelines recommend pulse oximetry in the immediate postoperative period, many argue that capnography may be a more reliable and sensitive predictor of hypoventilation and an earlier detector for potential respiratory adverse events (RAEs). Because it is a breath-by-breath monitor, capnography provides an earlier indication of impending respiratory compromise. What is the evidence? A study conducted by McCarter et al. (n=634) found that capnography was more effective than pulse oximetry in providing early warning of respiratory depression in postoperative patients receiving supplemental oxygen.9 In all cases, capnography but not pulse oximetry alerted the nurse that respiratory compromise was impending. It is this breath-by-breath monitoring that better predicted the need for intervention.
The American Society of Anesthesiologists (ASA)—Standards for Basic Anesthetic Monitoring, updated in 2010, now states that the adequacy of ventilation during general anesthesia and moderate/deep sedation shall be continually evaluated by both qualitative clinical signs and monitoring of expired carbon dioxide.10 This identifies the monitoring of expired carbon dioxide as a means to assess the adequacy of ventilation and has been implemented in part due to the risks associated with procedural sedation.10
Surrogates such as respiratory rate are measures that do not necessarily determine the adequacy of ventilation. A capnometer provides a quantitative measurement of the presence of exhaled carbon dioxide as well as a measure of the respiratory rate, though it does not provide information about the tidal volume. Carbon dioxide monitoring is required based upon the level of sedation, moderate or deep, and is irrespective of the location or type of anesthesia used. We would make an argument that a similar degree of monitoring should be maintained in the postoperative environment where most patients are still in a sedated state, especially in patients at high-risk for respiratory compromise.
In a small prospective randomized study of 54 opioid-naive postoperative orthopedic patients, capnography resulted in greater detection of respiratory depression.11 The authors concluded that capnography might be more appropriate for use with postsurgical high-risk patients taking opioids on a general care nursing unit.11 Concerns regarding this technology include consistent appropriate positioning of the end-tidal CO2 monitoring device in awake extubated patients, patient comfort, and less familiarity with this device compared to pulse oximetry by nursing staff. These issues can be addressed by both patient and nursing education.
In summary, improving patient safety and health care costs are two prominent goals of most policy change. The issue of adverse postoperative respiratory events has come center stage again as technological advancements allow for easy additional monitoring in the perioperative setting. Pulse oximetry has become standard of care in many areas outside of the operating rooms; we believe that postoperative capnography should also be adopted in the postoperative environment for continuous monitoring of end-tidal CO2 and earlier detection of catastrophic respiratory events.
Sofia Geralemou, MD Stony Brook University Hospital
Stephen Probst, MD Stony Brook University Hospital
Tong Joo Gan, MD, MHS, FRCA Professor and Chairman, Department of Anesthesiology Stony Brook School of Medicine
The State University of New York Stony Brook, NY
References
- Smetana GW, Lawrence VA, Cornell JE; American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006;144:581–95.
- Warner DO: Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology 2000; 92:1467–72.
- Zhan C, Miller MR: Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 2003; 290: 1868–1874.
- Arozullah, AM, Daley J, Henderson WG, Khuri SF: Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. Annals of Surgery 2000; 232.2: 242–253.
- Weingarten TN, Herasevich V, McGlinch MC, Beatty NC, Christensen ED, Hannifan SK, Koenig AE, Klanke J, Zhu X, Gali B, et al. Predictors of delayed postoperative respiratory depression assessed from naloxone administration. Anesth Analg 2015; 121:422–9.
- Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 2010;112:226–38.
- Krauss B, Hess D. Capnography for procedural sedation and analgesia in the emergency department. Annals of Emergency Medicine 2007; 50:172–181.
- Burton JH, Harrah JD, Germann CA, Dillon DC. Does end-tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices? Acad Emerg Med 2006;13:500–4.
- McCarter T, Shaik Z, Scarfo K, Thompson LJ. Capnography monitoring enhances safety of postoperative patient-controlled analgesia. American Health and Drug Benefits 2008;28–35.
- ASA Standards for Basic Anesthetic Monitoring, Standards and Practice Parameters (www.asahq.org – Last accessed August 9, 2016.)
- Hutchison R, Rodriguez L: Capnography and respiratory depression. The American Journal of Nursing 2008;108: 35–43.


 Issue PDF
Issue PDF