Anesthesia practice regulations, some of which have been in existence for quite a long time, help guide anesthesia care in Switzerland and the Federal Republic of Germany.
By way of background, note that in 1978, the Health Council of the Netherlands submitted to the Minister and the State Secretary for Health and Environmental Protection an Advisory Report on Anesthesiology, which was published in 1980. This report contained regulations covering the provision of anesthesiological workplaces with monitoring equipment @ In October 1986, the ASA issued their Standards for Basic intra-operative Monitoring! Following this, the national societies or associations of Canada (1987), Australia (1988), Singapore (I 988), United Kingdom and Ireland (I 988), Belgium (1989) and France (1989) adopted and issued 1,2,1 recommendations for Monitoring during anesthesia.
In August 1986, a Committee of the Swiss Society of Anesthesiology and Reanimation adopted Guidelines for Monitoring in Anesthesia. These stipulate that the equipment required for minimal monitoring must be considered a standard provision for the conduction of an anesthetic, and must consist of:
a) ECG monitoring.
b) Disconnected alarm that gives an audible signal.
c) An 02 monitor with a lower limit alarm (inspiratory 02 concentration measurement or pulse -oximetry).
d) Equipment for the monitoring of temperature and muscle relaxation (peripheral nerve stimulator) must be available
e) For cardiopulmonary resuscitation, appropriate equipment, including a defibrillator, must be made available within an acceptable tune
f) In every hospital, facilities for the following examinations must be available on a 24-hour basis: ECG, chest X-ray, blood gas analysis, hemoglobin/hematocrit, potassium, creatinine, blood sugar, coagulation.
g) The following devices are identified as useful and desirable for anesthesia: C02 monitor, automatic sphygmomanometer, pulse oximeter, blood-warming machine.
Equivalent to the standards recommended by the ANSI, a Swiss Standard (SN 057 600) has been available since 1987 entitled Inhalation Anesthesia Systems Employing Continuous Flow for Use in Human Medicine. An 02 analyzer must be incorporated within the anesthesia system. This device should he provided with an adjustable low concentration limit alarm that gives both an optical and acoustic signal. A pressure gauge in the breathing circuit is recommended.
German Efforts
Since June 1984, the German Standard (DIN 13253) Inhalation Anesthesia Machines Technical Safety Requirements and Testing has been available in the Federal Republic of Germany. Virtually identical with this is the Standard OENORM K 2003 issued in Austria in June 1988. The following monitoring devices are required:
a) An 02 concentration measuring device incorporated within the inspiratory limb of the breathing system provided -with an adjustable low concentration limit alarm that gives an audible signal.
b) Pressure gauge in the breathing system.
c) For automatic ventilators, a disconnect and a stenosis (excessive pressure) alarm.
d) A spirometer in the expiratory limb if a circle system is employed
e) If the ventilation pressure and volume cannot be measured owing to the mode of function of the anesthetic equipment, alternative procedures must be employed, for example, end-expiratory C02 monitoring.
Agent Monitoring Required
 In January 1985, the government of the Federal Republic of Germany issued a Decree on the Safety of Medical-technical Equipment (MedGV). In this, it is required that the devices involving the dosed application of energy or medications must be provided with an alarm facility that is activated in the event of a device related dosaging error. Since inhalation anesthetic machines fall within this regulation, from January 1, 1988, they must be provided with a facility for measuring the amount of a volatile anesthetic delivered by the vaporizer.
In January 1985, the government of the Federal Republic of Germany issued a Decree on the Safety of Medical-technical Equipment (MedGV). In this, it is required that the devices involving the dosed application of energy or medications must be provided with an alarm facility that is activated in the event of a device related dosaging error. Since inhalation anesthetic machines fall within this regulation, from January 1, 1988, they must be provided with a facility for measuring the amount of a volatile anesthetic delivered by the vaporizer.
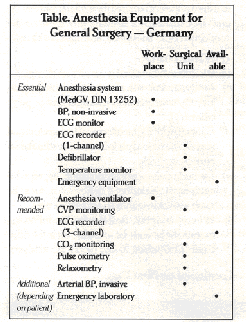
In October 1989, the German Society of Anesthesiology and Intensive Care Medicine, together with the Professional Association of German Anesthetists issued their Guidelines for Quality Assurance in Anesthesiology. (6) These make a differentiation between the organizational requirements (process quality) and spatial, equipment, and staff requirements(structure quality)of quality assurance. As in the case of the guidelines employed in the Netherlands, any attempt to stipulate what devices must be employed for given operations or anesthetic techniques was deliberately avoided. What is stipulated, however, is what devices have to be available for the specific task in hand. Whether and when such devices are employed is left to the decision of the anesthesiologist, who is required to proceed in accordance with the requirements and circumstances of the individual case.
The table lists the equipment applicable to the area of general surgery. In the case of a non-operative workplace(for example diagnostic, obstetric), CVP measurement, three-channel recording of the ECG, relaxometry and invasive arterial pressure measurement can all be left out. This also applies to the area of outpatient anesthesia, for which C02 monitoring, too, is not mandatory. For workplaces in the field of pediatric surgery, chest surgery and surgery on the major arteries, cardiac surgery and neurosurgery, additional requirements are needed, which, for reasons of space, cannot be listed here. However, copies of all the guidelines and recommendations mentioned in this paper can be requested from the author.
Thomas Pasch, M. D., is Professor Anesthesiology and Chairman of the Institute of Anesthesiology at the University Hospital, Zurich, Switzerland.
References
1. Adams AP. Recommendations for monitoring standards in the U.K. and Ireland. APSF Newsletter 1989; 4:32-3. 2. Belgium standards for patient safety in anaesthesia. Acta Anaesthesiol US 1989; 40; in press.
3. Crul T. The Netherlands national approach to standards of safety and care in anesthesia. Eur J Anesthesiol 1987; 4:213-5.
4. Eichhorn, [H. ASA adopts basic monitoring standards. APSF Newsletter 1987; 2:1-3.
5. Faculty of Anesthetists, R.A.C.S. Statement June 1988. Monitoring during anaesthesia (Australia). APSF Newsletter 1988; 3:23.
6. Quality assurance in anesthesiology. Guidelines of the German Society of Anesthesiology and Intensive Care Medicine and of the Professional Association of German Anesthetists (in German). Anaesth Intensiv med 1989; 30:307-14.

