Summit Addresses Intravenous Safety
Intravenous medications have saved the lives of millions of patients. However, partly because of the huge number of doses and the number of different medications given daily, errors in IV medication administration still represent a significant health care problem in the United States today. It has been almost 9 years since the Institute of Medicine’s Report “To Err is Human” shocked the public consciousness and the medical establishment in 1999.1 Since then, much has been said and written about the problem, and there have been some significant steps forward. The Joint Commission has made reduction of medical errors one of its national patient safety goals for the past several years. In 2006, the FDA mandated that manufacturers include a machine readable bar code on the label of the containers of many prescription drugs. New technology such as bar codes, radio frequency identification (RFID), and computerized order entry (CPOE) systems have all come on the horizon as technological solutions, only to create different problems which may be almost as big as those they are intended to solve.
It is painfully obvious that we are as yet nowhere near a solution, even for the so called “high alert” medications! The administration of flush solution with a heparin concentration 1000-times that intended to 17 Texas neonates in Corpus Christi on July 4 this year is just one very recent example of how far we have to go.
According to the 2006 Institute of Medicine Report “Preventing Medical Errors,” on average, a hospitalized patient is subject to at least 1 medication error per day, with at least 1.5 million preventable adverse drug reactions occurring each year. These reactions lead to an estimated $3.5 billion in additional health care costs annually to hospitalized patients alone, and reactions to drugs represent between the fourth and sixth leading cause of deaths in hospitalized patients.
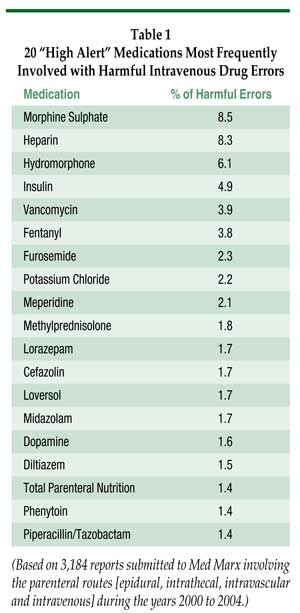
According to an analysis of over 73,000 intravenous drug errors reported to the US Pharmacopoeia MedMarx database between 2000 and 2004, more than 50% of errors were in the process of actually administering medications, and 60% of these errors occurred in the intravenous administration of 1 of 20 “high alert” medications (Table 1). Between 3% and 5% of these reported errors led to patient harm.
In the MedMarx database, one-half of errors occurred on patient units or nursing floors. However, 5% occurred in the operating room or in the pre- or post-anesthesia care units, where anesthesiologists and nurse anesthetists routinely practice. In the operating room, it has been estimated that 1 drug administration error occurs for every 133 anesthetics.2 Approximately 1% of these errors actually cause patient harm. Therefore, elimination of medication errors represents a tremendous opportunity to save lives and improve patient care in the OR as well as in the remainder of the hospital.
In order to save lives and prevent harm to patients, far reaching changes are needed in the way medications are prepared and administered. The persistence of the problem has led to a new sense of urgency on the part of many organizations dedicated to patient safety, including the Institute of Medicine (IOM), the Institute for Safe Medical Practice (ISMP), Emergency Care Research Institute (ECRI), Joint Commission, Food and Drug Administration (FDA), Centers for Medicaid and Medicare Services (CMS), National Patient Safety Foundation (NPSF), United States Pharmacopeia (USP), Agency for Healthcare Research and Quality (AHRQ), the Institute For Healthcare Improvement (IHI), the National Quality Forum (NQF), and many other professional and specialty organizations.
Representatives of these and other national organizations joined in an intensive 2-day Intravenous Safety Summit convened by the American Society of Health System Pharmacists (ASHP) in Rockville, MD, on July 14-15 to recommend very specific and attainable changes in practice that will be effective in preventing medication errors and saving lives now lost to adverse drug events. One of the real strengths of this summit was the integral involvement of frontline practitioners—nurses, physicians, and pharmacists, as well as vendors, health system experts, and researchers. Three participants in the summit represented the specialty of anesthesiology: Jeffrey B. Cooper, PhD, represented the Anesthesia Patient Safety Foundation; Donald E. Martin, MD, represented the American Society of Anesthesiologists; and Nathaniel M. Sins, MD, represented Partners Healthcare and brought unique expertise in the emerging “smart pump” technology.
Specific recommendations from the summit are yet to be finalized. However, the themes for these recommendations were clear from the discussion and included
- The standardization of infusion concentrations and the units or format used to order or prescribe intravenous medication infusions (such as mcgm/min vs. mcgm/kg/min).
- Simplifying the administration process, with preference for the preparation of intravenous medication in the pharmacy rather than at the point of care.
- Obtaining the maximum benefit from technology in the form of bar codes, computerized order entry, and smart pumps.
- Establishing a culture conducive to medication safety.
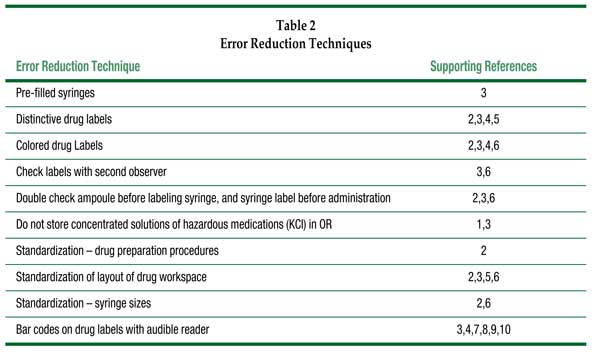
The specialty of anesthesiology and the practice of nurse anesthesia are both in very good positions to take leading roles in research, practice changes, and cultural changes needed to save lives now lost due to medication errors. Anesthesiologists are one of the few groups of physicians who are personally responsible for drug administration. Historically, our specialty has been able to effectively design monitors and ventilation systems and to greatly reduce death due to hypoxia or ventilatory failure in anesthetized patients. Anesthesiologists already have effective patient safety, “standards,” and “practice advisory” infrastructures in place. Significant research has already been done to evaluate drug administration procedures and technology to improve safety of drug administration during anesthesia (Table 2). All of these improvements have a potential to improve the process of drug administration in the operating room, which is a complex collection of more than 40 steps, if used to enable anesthesia providers to work more safely, as well as more quickly and efficiently.
Establishing a “culture of safety,” however, may be more difficult than developing technology. Stabile, Webster, and Merry, in the Fall 2007 issue of the Anesthesia Patient Safety Foundation Newsletter, called for just such a cultural shift in medicine, from a culture of productivity to a culture of safety. In their words, “safety should be funded because it is the right thing to do, not because of any ROI directives.” Commercial aviation and other similarly complex yet high-risk industries adopted a culture of safety years ago. Medicine can do no less.
Therefore, as a very important next step, the Anesthesia Patient Safety Foundation is planning a Board of Directors’ Workshop for Friday, October 17 in Orlando, FL, entitled “Innovations in Medication Safety in the Operating Room.” The workshop is designed to identify solutions for medication errors in the operating room that are currently technologically feasible, as well as ideas for potential new processes to be developed and explored. Participants in this workshop will include physicians, pharmacists, health systems and technology researchers, as well as representatives from the Joint Commission and other regulatory agencies. The very practical solutions coming from this workshop, as well as the ASHP IV Medication Safety Summit may well provide the innovations in drug administration processes, as well as insight into the human factors responsible for inevitable human errors, which are needed to bring about a true reduction in medication errors in the operating room.
Dr. Martin is Professor of Anesthesia at Penn State University College of Medicine in Hershey, PA. Dr. Martin is also Chair of the ASA Committee on Equipment and Facilities.
References
- Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Committee on the Quality of Health Care in America, Institute of Medicine. Washington, DC: National Academy Press, 1999.
- Fasting S, Grisvold S. Adverse drug errors in anesthesia, and the impact of coloured syringe labels. Can J Anaesth 2000;47:1060-7.
- Jensen LS, Merry AF, Webster CS, et al. Evidence-based strategies for preventing drug administration errors during anaesthesia. Anaesthesia 2004;59:493-504.
- Abeysekera A, Bergman IJ, Kluger MT, et al. Drug error in anaesthetic practice: a review of 896 reports from the Australian Incident Monitoring Study database. Anaesthesia 2005;60:220-7.
- Orser BA, Oxorn DC. An anaesthetic drug error: minimizing the risk. Can J Anaesth 1994;41:120-4.
- Currie M, Mackay P, Morgan C, et al. The “wrong drug” problem in anaesthesia: an analysis of 2000 incident reports. Anaesth Intensive Care 1993;21:596-601.
- FDA Issues Bar Code Regulation. February 25, 2004: United States Food and Drug Administration. Available at: http://www.fda.gov/oc/initiatives/barcode-sadr/fs-barcode.html. Accessed August 25, 2008.
- Kaushal R, Bates DW. Information technology and medication safety: what is the benefit? Qual Saf Health Care 2002;11:261-5.
- Merry AF, Webster CS, Mathew DJ. A new, safety-oriented, integrated drug administration and automated anesthesia record system. Anesth Analg 2001;93:385-90.
- Merry AF, Webster CS, Weller J, et al. Evaluation in an anaesthetic simulator of a prototype of a new drug administration system designed to reduce error. Anaesthesia 2002;57:256-63.


 Issue PDF
Issue PDF