Episode #96 Pediatric Patient Safety Threat: Button Battery Ingestion
May 3, 2022Welcome to the next installment of the Anesthesia Patient Safety podcast hosted by Alli Bechtel. This podcast will be an exciting journey towards improved anesthesia patient safety.
Our featured article is “Perioperative Management of Button Battery Ingestions in Children” by Hoagland, Yee, Ing, and Chatterjee.
Head over to the National Capital Poison Center for detailed management guidelines. You can find these guidelines here. www.poison.org/battery/guideline
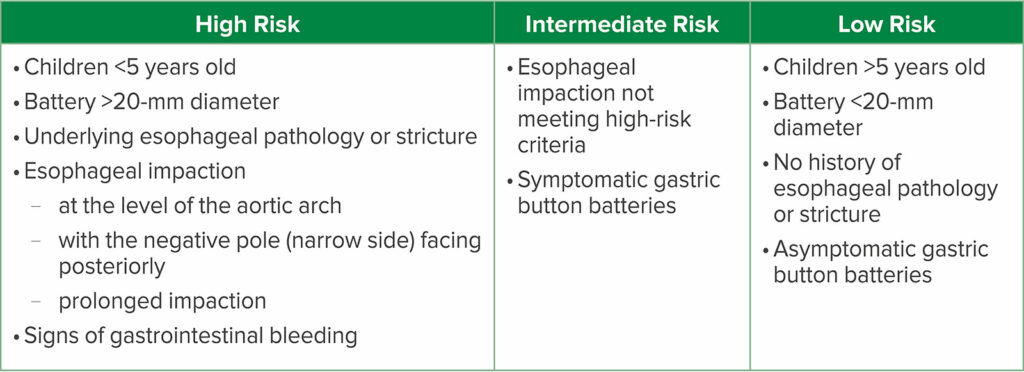
Check out Table 1 in the article to learn more about risk stratification for children with button battery ingestions. This is important once the decision has been made to proceed with emergent endoscopic removal. Remember, high-risk patients may require additional specialized care and resources.
Table 1: Risk Stratification for Button Battery Ingestions in Children9
Check out these two publications for further information about anesthetic management and considerations for patients with button battery ingestion.
- Hoagland MA, Ing RJ, Jatana KR, et al. Anesthetic implications of the new guidelines for button battery ingestion in children. Anesth Analg. 2020;130:665–672.
- Eck JB, Ames WA. Anesthetic implications of button battery ingestion in children. 2020;132:917–924.
Be sure to check out the APSF website at https://www.apsf.org/
Make sure that you subscribe to our newsletter at https://www.apsf.org/subscribe/
Follow us on Twitter @APSForg
Questions or Comments? Email me at [email protected].
Thank you to our individual supports https://www.apsf.org/product/donation-individual/
Be a part of our first crowdfunding campaign https://www.apsf.org/product/crowdfunding-donation/
Thank you to our corporate supporters https://www.apsf.org/donate/corporate-and-community-donors/
Additional sound effects from: Zapsplat.
© 2022, The Anesthesia Patient Safety Foundation
Hello and welcome back to the Anesthesia Patient Safety Podcast. My name is Alli Bechtel, and I am your host. Thank you for joining us for another show. We are on a roll discussing articles from the February 2022 APSF Newsletter and there is more to discuss. This time we are heading over to the nearest Children’s hospital or pediatric ward because our featured article today is all about a serious safety threat for pediatric patients.
Before we dive into the episode today, we’d like to recognize Merck, a major corporate supporter of APSF. Merck has generously provided unrestricted support to further our vision that “no one shall be harmed by anesthesia care”. Thank you, Merck – we wouldn’t be able to do all that we do without you!”
Our featured article is “Perioperative Management of Button Battery Ingestions in Children” by Hoagland, Yee, Ing, and Chatterjee. To follow along with us, head over to APSF.org and click on the Newsletter heading. First one down is the current issue. Then, scroll down until you get to our featured article today. I will include a link in the show notes as well. You can also get to the February 2022 APSF Newsletter by clicking on the Newsletter heading and 5th one down is the Newsletter archives and then scroll down until you get to the February 2022 APSF Newsletter. This is also where you can find all of the APSF Newsletters all the way back to 1986.
Before we get into the article, we are going to here from one of the authors. Let’s take a listen.
[Chatterjee] “Hello, my name is Deb Chatterjee and I’m a pediatric anesthesiologist at Children’s Hospital Colorado and the University of Colorado. I also represent the American Academy of Pediatrics Section of Anesthesiology at the National Button Battery Taskforce. I’m speaking on behalf of my co-authors and colleagues, Drs. Monica Hoagland, Sydney Yee, and Richard Ing all from Children’s Hospital Colorado.
[Bechtel] To kick off the show, I asked Chatterjee, why he feels so passionate about this topic. Here is what he had to say.
[Chatterjee] “Button battery ingestions are extremely dangerous especially in young children. It is estimated that more than 3,500 button battery ingestions are reported to the National Poison Control Center each year and the number of major complications and deaths in children has increased more than 7-fold since the introduction of the 20 mm CR 2032 lithium batteries in 2006. In fact, button battery ingestion in children less than 6 years of age result in major complications in about 12% of the cases. Therefore, early diagnosis and immediate endoscopic removal of these ingested button batteries is critical.”
[Bechtel] And now it’s time to get into the article. The authors start off by highlighting the scope of the problem. Have you taken care of a patient with a foreign body ingestions? This is definitely something that anesthesia professionals need to be aware of since foreign body ingestions are common for pediatric patients. When it comes to foreign body ingestions, button battery ingestion is a big cause for concern. The 3-volt-20mm lithium batteries became available in 2006. Since that time, the rates of emergency department visits, significant morbidity, and mortality from button battery ingestions have increased since this newer battery is more powerful leading to increased esophageal impaction and tissue injury. If we look at the data, the overall incidence of significant morbidity and mortality following button battery ingestion is 0.42%. If we look a little closer, the rates for significant complications for children younger than 6 years old following button battery of greater than 20mm ingestion is over 12%. Children younger than 5 years old are particularly vulnerable and this is the age group with all the reported fatalities.
Why is button battery ingestion so bad? The battery causes an electrolytic current that hydrolyzes tissue fluids with the production of hydroxide ions at the battery’s negative pole. When this happens, there is the creation of a very alkaline environment with local tissue pH as high as 12-13 with the resultant liquefactive necrosis of the surrounding tissues. The damage may be extensive with perforation and erosion into nearby structures such as the airway, major blood vessels, mediastinal structures or spinal cord. The most common cause of mortality is hemorrhage from esophageal-vascular fistulae or complications from a tracheoesophageal fistula. Patients who develop an aorto-esophageal fistula are at high risk for mortality. There are only 4 cases of patients surviving this complication in the literature.
For these cases, quick and appropriate triage and management is necessary for confirmed or suspected button battery ingestion. The extent of the injury and risk of complications depends on the following factors:
- Location since esophageal impactions are associated with longer contact between the battery and esophageal tissue with an increased risk for significant tissue damage.
- Duration of impaction
- Plus, the orientation, size, and voltage of the button battery
Anesthesia professionals need to be prepared to act quickly to help keep these patients safe since tissue damage may start within 15 minutes of contact with increasing risk of morbidity and mortality with increased duration of exposure. The liquefactive necrosis continues even after the button battery has been removed. As you can, these patients require emergent endoscopy to remove the battery as soon as possible or within 2 hours of ingestions and patients will need to be monitored closely after the procedure for worsening injury.
This 2 hour time frame may be difficult since the diagnosis may be delayed for several reasons including:
- Unwitnessed foreign body ingestions in children
- Non-specific signs and symptoms which may mimic respiratory or gastrointestinal illnesses
- Lack of emergency treatment by parents and emergency treatment
- Lack of availability of pediatric specialists and the necessary equipment.
Patients may need multiple pediatric specialists to help provide care including otolaryngologists, gastroenterologists, general or cardiothoracic surgeons, and of course anesthesia professionals. For patients who present to rural hospitals without these physicians and the equipment available, emergent transfer is required, but there may be a significant delay in removing the button battery. There are standardized protocols for initial triage and management of patients with suspected button battery ingestion to help identify high risk patients and expediate removal of the battery. Head over to the National Capital Poison Center for detailed management guidelines and I will include the link in the show notes.
Let’s go through the triage and management now. First, initial evaluation should include neck, chest, and abdomen x-rays to identify and locate the foreign body. Emergent removal is required for esophageal foreign bodies, symptomatic gastric button batteries, and combination ingestions of battery and magnet. Conservative management may be appropriate for asymptomatic patients older than 12 years old with no history of esophageal pathology, with a known ingestion of a single small battery that is smaller than 12 mm in diameter.
It’s time to talk about Table 1 from the article which reviews risk stratification for patients with known or suspected button battery ingestions and provides important information for patients who require emergent endoscopic removal of the button battery. Let’s review it now. In the low-risk category, we have the following: Children over 5 years of age, Battery smaller than 20mm in diameter, no history of esophageal pathology or stricture, asymptomatic, gastric location of the button battery. If we move up to the Intermediate Risk group, this includes esophageal impaction not meeting the high-risk criteria as well as symptomatic gastric button battery. Finally, let’s look at the patients that are the highest risk. This group includes children less than 5 years old who ingest a battery larger than 20 mm in diameter, underlying esophageal pathology or stricture, evidence of esophageal impaction including location at the level of the aortic arch with the negative pole facing posteriorly and prolonged impaction, and finally signs of gastrointestinal bleeding since this may be a sign of a vascular-esophageal fistula. Keep in mind that the negative pole is the narrow side of the battery and when this is facing posteriorly there is an increased risk for vascular injury.
Patients in the intermediate and low-risk categories may undergo the emergent endoscopy in a general operating room by gastroenterologists with or without general surgeons on standby. High risk patients may require additional specialist care and available resources including interventional cardiologists or cardiothoracic surgeons. Management for these patients may include invasive vascular access, additional hemodynamic monitoring, resources for volume resuscitation, and blood product administration.
It is time to review considerations for intraoperative and postoperative anesthetic management. You will need to be prepared for hemodynamic and/or respiratory complications from vascular or airway tissue damage. Communication is vital so that everyone is prepared when the battery is removed. In addition, after the battery is removed, repeat endoscopy and bronchoscopy are required to evaluate the esophagus and airway for additional injury. Airway management should include a rapid sequence induction. Make sure you have adequate IV access for fluid resuscitation if needed as well as appropriate monitors which may include invasive monitors. During the postoperative period, patients need close monitoring for further injury to the esophagus and surrounding tissues. The length of stay and level of care depends on the initial injury and evaluation when the battery was removed. Keep in mind that patients may need serial imaging as well as additional anesthesia care during repeat endoscopic evaluation.
We have so much more to talk about on this topic, but we are almost out of time for today. We hope that you will tune in next week as we review anesthetic considerations and mitigation strategies for patients with button battery ingestion. We are also going to talk about the updated management guidelines from the National Poison Control Center. Before we wrap up for today, we are going to hear from Chatterjee once again. I asked him, “What do you hope to see going forward.” This is what he had to say.
[Chatterjee] “Recently, the National Poison Control Center updated their management guidelines to include the preoperative administration of honey or Carafate to neutralize the strong alkaline reaction in children more than 12 months of age for suspected lithium battery ingestion within the prior 12 hours. It is important to note that administering honey is not a substitute for immediate removal, and these cases must not be delayed for NPO status. One of our fellows, Dr. Sydney Yee, created a couple of infographics one for parents to increase awareness about the dangers of button battery ingestions in young children and another infographic for anesthesia providers to help identify high risk patients and highlight the major anesthetic considerations. By increasing awareness about this important issue, we hope that button battery ingestions can be both prevented and better managed.”
[Bechtel] Thank you so much to Chatterjee for contributing to the show today. We hope that you will check out these infographics and share them with parents of young children as well as any anesthesia and other healthcare professionals that you work with who may be involved in the care of pediatric patients following button battery ingestions and tune in next week because we will be continuing our discussion on the perioperative management of button battery ingestion in children.
If you have any questions or comments from today’s show, please email us at [email protected]. Please keep in mind that the information in this show is provided for informational purposes only and does not constitute medical or legal advice. We hope that you will visit APSF.org for detailed information and check out the show notes for links to all the topics we discussed today.
If you have not done so already, we hope that you will rate us and leave a review on iTunes or wherever you get your podcasts and feel free to share this podcast with your friends and colleagues and anyone that you know who is interested in anesthesia patient safety. Plus, you can let us know that you are listening by tagging us @APSForg using the hashtag #APSFpodcast.
Until next time, stay vigilant so that no one shall be harmed by anesthesia care.
© 2022, The Anesthesia Patient Safety Foundation