Episode #249 Sugammadex Safety: Special Populations, Special Concerns
April 9, 2025Welcome to the next installment of the Anesthesia Patient Safety podcast hosted by Alli Bechtel. This podcast will be an exciting journey towards improved anesthesia patient safety.
Our featured article today is “Safety of Sugammadex in Pregnancy, Pediatrics, and Renal Failure” by Kevin Yang, Christina Ratto, Joseph Szokol, and Ashley Osumi.
Here is the citation for the article that we discussed on the show today:
- Oh MW, Mohapatra SG, Pak T, et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade in patients with severe renal impairment: a randomized, double-blinded study. Anesth Analg. 2024;138:1043–1051. PMID: 38190344.
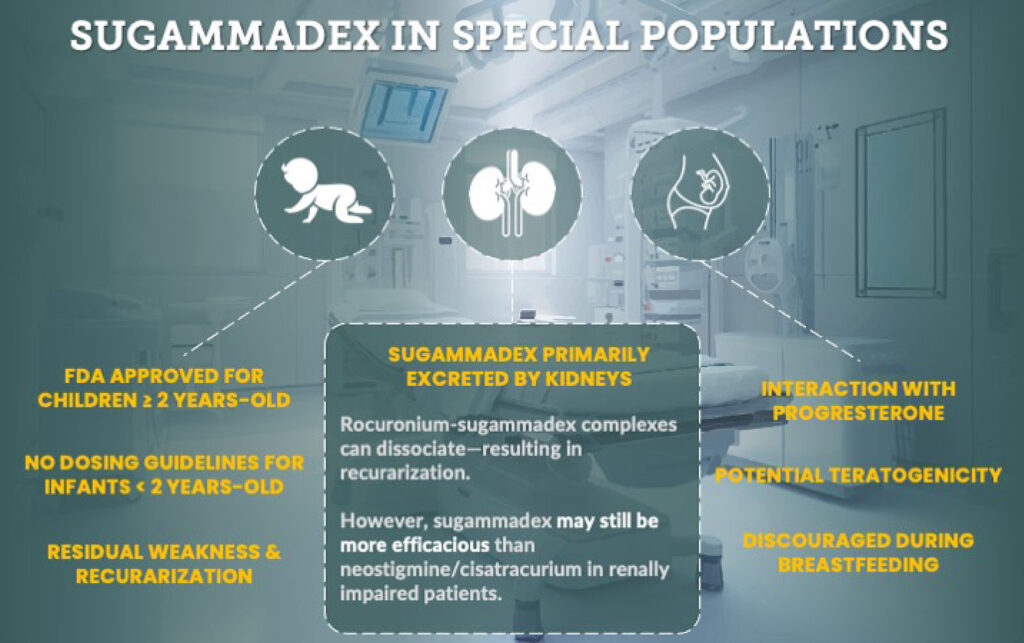
Spoiler Alert!! This infographic sums up the safety considerations for special populations that are covered in the article. Today, we are covering patients with renal impairment and pregnant patients. We will be talking about pediatric patients on the show next week.
Did you know that the Manual External Defibrillation, Cardioversion, and Pacing Technology Education Initiative is now available? This course offers eight topics to help you develop knowledge and skills to care for patients experiencing life threatening advanced cardiovascular life support events requiring the use of a manual external defibrillator. Plus, you can encourage your colleagues, trainees, and perioperative team members to complete the course as well. This course is available at no cost and delivered through the ASA learning management system? Check it out here.
Click here to take the Manual External Defibrillation, Cardioversion, and Pacing course on the ASA website
(This link navigates away from the APSF website to the ASA website to sign-in and access the course.)
Courses are available to any learner at no cost. All courses are delivered through the ASA learning management system.
This episode was edited and produced by Mike Chan.
Subscribe to our YouTube Channel here: https://www.youtube.com/@AnesthesiaPatientSafety
Be sure to check out the APSF website at https://www.apsf.org/
Make sure that you subscribe to our newsletter at https://www.apsf.org/subscribe/
Follow us on Twitter @APSForg
Questions or Comments? Email me at [email protected].
Thank you to our individual supports https://www.apsf.org/product/donation-individual/
Be a part of our first crowdfunding campaign https://www.apsf.org/product/crowdfunding-donation/
Thank you to our corporate supporters https://www.apsf.org/donate/corporate-and-community-donors/
Additional sound effects from: Zapsplat.
© 2025, The Anesthesia Patient Safety Foundation
Hello and welcome back to the Anesthesia Patient Safety Podcast. My name is Alli Bechtel, and I am your host. Thank you for joining us for another show. We are returning to the February 2025 APSF Newsletter. Our featured topic involves a newer anesthesia medication, Sugammedex and some important patient safety considerations. We are still learning more about this medication, so stay tuned.
Before we dive further into the episode today, we’d like to recognize Fresenius Kabi, a major corporate supporter of APSF. Fresenius Kabi has generously provided unrestricted support to further our vision that “no one shall be harmed by anesthesia care”. Thank you, Fresenius Kabi –we wouldn’t be able to do all that we do without you!”
Our featured article today is “Safety of Sugammadex in Pregnancy, Pediatrics, and Renal Failure” by Kevin Yang, Christina Ratto, Joseph Szokol, and Ashley Osumi. To follow along with us, head over to APSF.org and click on the Newsletter heading. First one down is the Current Issue. Then, scroll down until you get to our featured article today. I will include the link in the show notes as well.
To help kick off the show today, we are going to hear from one of the authors. I will let him introduce himself now.
[Kevin Yang] “My name is Kevin Yang and I’m a current fourth year medical student at the Keck School of Medicine of USC in Los Angeles, California.”
[Bechtel] I asked Yang, what got him interested in this topic? Let’s take a listen to what he had to say.
[Yang] The reason I became interested in this topic is my mother, who is a recently retired anaesthesiologist. For most of her practice, succinylcholine was the preferred paralytic agent because it wears off so quickly, making ending a case pretty easy. However, most of the listeners will know that succinylcholine has some well documented potential adverse effects.
Now, a major shift in practice since then has been the introduction of Sugammadex, which quickly and reliably reverses the neuromuscular blockade of non-depolarizing agents, such as rocuronium, agents which do not have the adverse effects associated with succinylcholine. The use of Sugammadex and the way it has changed the practice of anaesthesia since my mom practiced was a topic that piqued my interest.
So, I was excited when I had the opportunity to write this literature review investigating the safety of this relatively new drug.”
[Bechtel] Thank you to Yang for helping introduce this topic. Do you remember the first time you used Sugammadex in clinical practice? Did it feel like an almost magical reversal agent? Sugammadex offers a quick and reliable reversal from neuromuscular blockade, but lucky for us, it is not magic. It’s science and today, we are talking about the safety considerations.
We have talked about the 2023 American Society of Anaesthesiologists practice guidelines for the monitoring and antagonism of neuromuscular blockade on the podcast before. Remember, these guidelines recommend using quantitative monitoring instead of qualitative monitoring to help prevent residual blockade. Another recommendation is for the use of Sugammadex rather than Neostigmine depending on the depth of blockade. The question is, can we use Sugammadex for all patients? What about patients with renal failure, pregnant patients, and paediatric patients?
Let’s check out Figure 1 in the article for consideration of Sugammadex in Special Populations. First for pediatric patients, Sugammadex is approved for Children 2 years old and up, there are no dosing guidelines for infants less than 2 years old, and there is a risk for residual weakness and recurarization in this patient population. Next up, patients with renal failure. Did you know that Sugammadex is primarily excreted by the kidneys? Plus, rocuronium-sugammadex complexes can dissociate leading to recurarization, but keep in mind that sugammadex may still be more effective than neostigmine to reverse cisatracurium in renally impaired patients. Finally, for pregnant patients, sugammadex interacts with progesterone and has a potential for teratogenicity. The use of sugammadex is discouraged during breastfeeding.
Now that we have shown you the trailer with all the spoilers, let’s take a closer look at these special patient populations, starting with the safety of sugammadex in renal failure. Here we go. Sugammadex is primarily excreted by the kidney. This means that for patients with severe renal impairment, there is a risk of recurarization, which may occur when circulating rocuronium-sugammadex complexes disassociate. In patients with normal renal function, the elimination half-life of Sugammadex is about 2 hours with an estimated plasma clearance of about 88mL/min. Over 90% of the dose is excreted within 24 hours with 96% excreted unchanged in the urine. There is an increased half-life of 4, 6, and 19 hours in mild, moderate, and severe cases of renal impairment. Rocuronium and sugammadex form a very stable complex due to intermolecular van der Waals forces, thermodynamic hydrogen bonds, and hydrophobic interactions. Did you know that for every 25 million sugammadex-rocuronium complexes, there is only 1 complex that dissociates? The complex is water-soluble and excreted in the urine in patients with normal renal function and removed by dialysis with a high-flux filter.
Patients with severe renal dysfunction who are not on dialysis may be at risk since the rocuronium-sugammadex complex stays in the plasma longer meaning that there may be higher rates of disassociation. If you provide anaesthesia care for patients with renal dysfunction, what is your plan for paralysis and reversal? One option is to administer a neuromuscular blocking agent and then wait for recovery of function. Another option is to use cisatracurium which is not reversible with sugammadex. And now it’s time to check out the literature. There was a recent prospective, randomized, blinded, and controlled trial that compared sugammadex and neostigmine for reversing moderate blockade in patients with renal impairment. This is the 2024 study published in Anesthesia and Analgesia. Check out the show notes for the citation. The results revealed that sugammadex administration led to a train of four ratio greater than 90% significantly faster than neostigmine without major adverse events. It is likely that using Sugammadex to reverse moderate blockade is safe and faster than the combination of neostigmine and cisatracurium and don’t forget to use a quantitative neuromuscular monitor to ensure that your patients with renal impairment are adequately reversed.
Now’s it’s time to move onto our next patient population so that you know what to expect when your patient is expecting. That’s right we are talking about the use of sugammadex in pregnancy.
Here’s what we know. There is no definitive data that demonstrates harm. At the same time, the Society for Obstetric Anesthesia and Perinatology, or SOAP, guidelines recommend against its use. These SOAP guidelines reveal the challenge in medical practice when there is a lack of conclusive drug safety data in pregnancy that leads to conservative recommendations and this may impact optimal management of pregnant patients who need neuromuscular blockade reversal.
Now, its time for a literature review to find out we have learned from recent studies. Sugammadex has the potential to bind progesterone. The initial manufacturer’s model suggests potential binding to progestin which means that there may be a similar binding with progesterone. Follow-up in vitro studies have shown that sugammadex can bind to progesterone. Thus, in pregnant patients who need surgery, there is a concern that sugammadex can bind progesterone and thus decrease progesterone levels which are crucial for maintaining pregnancy.
The current preclinical data reveals the following:
- A study in first-trimester pregnant rats who received high-dose sugammadex 30mg/kg showed no decrease in endogenous progesterone levels or any affect on live birth or stillbirth rates.
- Another study in pregnant rabbits who underwent general anaesthesia with paralysis and reversal with sugammadex showed significantly decreased progesterone levels. However, all the rabbit pregnancies were successful without early births or stillbirths.
- A single case report describes a pregnant patient who had surgery for ovarian torsion and received sugammadex without any pregnancy complications or side effects.
- Going forward, there is a call for more data and a registry which would allow anaesthesia professionals to report on the use of sugammadex in pregnant patients to help better study the effect of sugammadex on pregnancy progression.
There is a concern that Sugammadex binding progesterone will affect obstetric outcomes. Decreased progesterone levels are associated with preterm labour and preterm premature rupture of membranes. There is a case series of 25 pregnant patients who received sugammadex during the antenatal period with no obstetric complications directly related to the sugammadex administration. The authors of that study attributed the lack of complications to the minimal placental transfer of sugammadex and its high affinity for rocuronium which may limit the progesterone binding. Keep in mind that sugammadex has a half-life of about 2 hours with clearance from the bloodstream within the first 48 hours so it is likely that any potential side effects from progesterone binding would be seen within that timeframe.
For patients requiring caesarean delivery with general anaesthesia, sugammadex has been shown to be safe and effective following rocuronium administration. There is limited evidence for the use of high-dose sugammadex for rescue reversal in the setting of a cannot-intubate/cannot-ventilate scenario after rapid sequence induction. Current guidelines recommend considering using sugammadex in this emergency situation since the risk of severe hypoxia outweighs the potential risks from sugammadex exposure.
There are additional concerns about teratogenicity that came from cell culture studies that resulted in neuronal apoptosis due to oxidative stress, but later studies on mice with mature blood-brain barriers did not demonstrate this effect from exposure to sugammadex. There are preclinical studies that demonstrated no adverse effects in pregnant rats while high doses ion New Zealand white rabbits led to decreased fetal body weight and bone ossification issues without malformations. There is no evidence of these effects occurring in humans.
Sugammadex molecules are large and polarized which may limit the drug’s ability to cross the blood-brain barrier as well as limit it’s excretion into breast milk. This is an important consideration when thinking about using sugammadex for reversal during caesarean section or for any patients who are breastfeeding. Infants with immature metabolism and renal function may have delayed clearance if exposure to Sugammadex from breast milk. There is one unpublished preclinical study in rats that demonstrated peak sugammadex levels in rat milk 30 minutes after administration with to adverse effects on offspring. There is currently no data on sugammadex in human breast milk. As a result, it is likely that breastfeeding should be delayed immediately after receiving sugammadex keeping in mind that peak concentrations way be one hour after delivery with the potential for increased passage into breast milk during the early postpartum period.
Going forward, more information is needed to ascertain the risks and safety profile of sugammadex use for pregnant and breastfeeding patients. This will help guide safe practices in obstetric and non-obstetric settings where the use of sugammadex may be necessary and beneficial.
We still have more to talk about in the article, but we are going to hear from the author once again. I also asked Yang, “What do you envision for the future with regards to this topic or area of anaesthesia care?”
[Yang] “As we discuss in our literature review, despite Sugammadex’s now widespread use, the data regarding its safety in renal failure patients, pregnant patients, and paediatric patients remains in its infancy. So, I hope that the body of evidence regarding the use of this drug in those groups continues to grow so that we can better understand whether or not those groups can benefit from this drug in a safe way.
[Bechtel] Thank you so much to Yang for contributing to the show today. We are looking forward to continuing to discuss the safety considerations for Sugammadex use in clinical practice next week. Spoiler alert, we will be talking about the use of Sugammadex for pediatric patients.
If you have any questions or comments from today’s show, please email us at [email protected]. Please keep in mind that the information in this show is provided for informational purposes only and does not constitute medical or legal advice. We hope that you will visit APSF.org for detailed information and check out the show notes for links to all the topics we discussed today.
Did you know that the Manual External Defibrillation, Cardioversion, and Pacing Technology Education Initiative is now available? What are you waiting for? This course offers eight topics to help you develop knowledge and skills to care for patients experiencing life threatening advanced cardiovascular life support events requiring the use of a manual external defibrillator. Plus, you can encourage your colleagues, trainees, and perioperative team members to complete the course as well. Did I mention that it is available at no cost and delivered through the ASA learning management system? Check out the show notes for a link to the course so that you can further develop your resuscitation skills to help keep patients safe.
Until next time, stay vigilant so that no one shall be harmed by anesthesia care.
© 2025, The Anesthesia Patient Safety Foundation