Episode #216 Guarding Against Opioid-Induced Respiratory Depression in Kids
August 21, 2024Welcome to the next installment of the Anesthesia Patient Safety podcast hosted by Alli Bechtel. This podcast will be an exciting journey towards improved anesthesia patient safety.
Our featured article today is from the June 2024 APSF Newsletter. It is “Opioid-Induced Respiratory Depression—Pediatric Considerations” by Tricia Vecchione, and Constance L. Monitto.
Thank you so much to Connie Monitto for contributing to the show again today.
Here are the key takeaways:
- This is a preventable complication
- Anesthesia professionals need to be able to identify high-risk patients
- Utilize opioid-sparing adjuncts
- Perform frequent sedation assessment
- Maintain vigilant monitoring
Monitoring goal: Preemptively identify opioid-induced respiratory depression with time to intervene and prevent a critical event.
Ideal respiratory monitor: Continuously and accurately measure oxygenation, respiratory rate, carbon dioxide tension, and airflow.
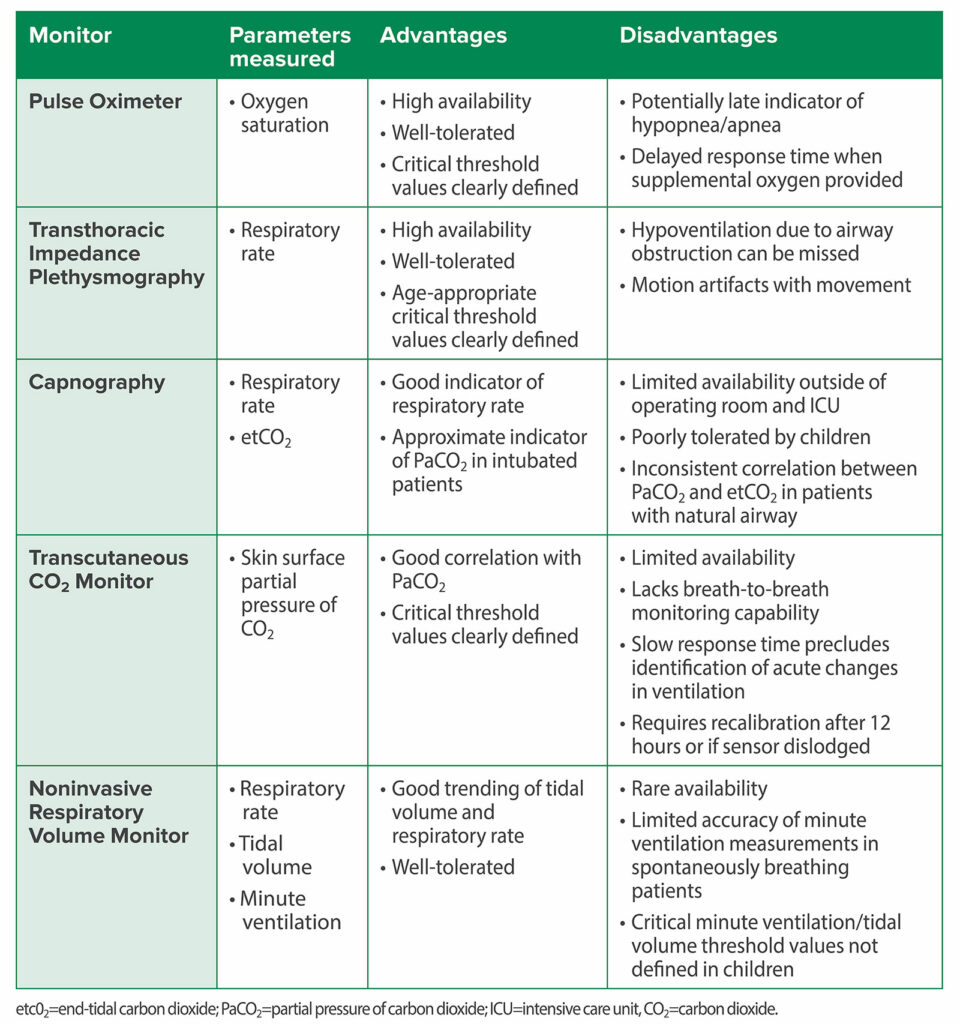
Check out Table 1 for a summary of available monitors.
Table 1: Summary of Respiratory Monitoring Modalities for Detection of OIRD.2,4,6,7,20-23
For more information and history of the pulse oximeter, check out this incredible APSF Resource, “Pulse Oximetry and the Legacy of Dr. Takuo Aoyagi.”
Here is the citation for the article that we talked about on the show today:
Miller KM, Kim AY, Yaster M, et al. Long-term tolerability of capnography and respiratory inductance plethysmography for respiratory monitoring in pediatric patients treated with patient-controlled analgesia. Paediatr Anaesth. 2015; 25:1054–1059. PMID: 26040512.
Subscribe to our YouTube Channel here: https://www.youtube.com/@AnesthesiaPatientSafety
Be sure to check out the APSF website at https://www.apsf.org/
Make sure that you subscribe to our newsletter at https://www.apsf.org/subscribe/
Follow us on Twitter @APSForg
Questions or Comments? Email me at [email protected].
Thank you to our individual supports https://www.apsf.org/product/donation-individual/
Be a part of our first crowdfunding campaign https://www.apsf.org/product/crowdfunding-donation/
Thank you to our corporate supporters https://www.apsf.org/donate/corporate-and-community-donors/
Additional sound effects from: Zapsplat.
© 2024, The Anesthesia Patient Safety Foundation
Hello and welcome back to the Anesthesia Patient Safety Podcast. My name is Alli Bechtel, and I am your host. Thank you for joining us for another show. We are returning to our conversation about opioid-induced respiratory depression in pediatric patients for Part 2 in our series. If you haven’t done so already, we hope that you will check out Part 1, Episode #215 as well.
Before we dive into the episode today, we’d like to recognize GE Healthcare, a major corporate supporter of APSF. GE Healthcare has generously provided unrestricted support to further our vision that “no one shall be harmed by anesthesia care”. Thank you, GE Healthcare – we wouldn’t be able to do all that we do without you!”
Our featured article again today is from the June 2024 APSF Newsletter. It is “Opioid-Induced Respiratory Depression—Pediatric Considerations” by Tricia Vecchione, and Constance L. Monitto. To follow along with us, head over to APSF.org and click on the Newsletter heading. The first one down is the current issue and from here, scroll down until you get to our featured article today. I will include a link in the show notes as well.
There is a call to action for anesthesia professionals address this threat to pediatric patient safety by identifying high-risk patients, using opioid-sparing adjuncts, performing frequent sedation assessment, and utilizing vigilant monitoring.
Let’s do a quick review. Here are the risk factors for increased risk of opioid-induced respiratory depression in pediatric patients:
- For Patient Factors, age less than one year of age and prematurity
- For Comorbidities, developmental delay, obstructive sleep apnea, respiratory dysfunction, neurological dysfunction, and obesity or underweight.
- For surgical factors, less than 24 hours after surgery and ENT surgery
- And for external factors, polypharmacy and supplemental oxygen.
Here are the monitoring considerations. Experts recommend continuous monitoring of respiratory rate and pulse oximetry for the first 24 hours unless the patient is awake and actively being observed for all pediatric patients who are started on parenteral opioids, who receive opioids by patient-controlled analgesia (PCA), or PCA by proxy, and/or continuous opioid infusion.
The Society for Pediatric Anesthesia provide additional recommendations for the use of perioperative opioids in children which include the following:
- Regular assessment of level of sedation using a validated sedation score that evaluates the patient’s level of alertness. This is an important assessment that is different from using a scale to monitor procedural sedation. You may consider using the Pasero opioid sedation scale.
- When starting opioid therapy for infants younger than 3 months of age, admission to a highly monitored environment such as the ICU, PACU, or step-down unit.
- Continuous monitoring of respiratory rate and electrocardiogram for pediatric patients receiving supplemental oxygen therapy. Remember, supplemental oxygen may decrease the sensitivity and response time of pulse oximetry as a monitor for apnea or hypopnea.
And now it’s time to return to the article. Let’s look a little closer at the options for pediatric respiratory monitoring and the challenges when it comes to monitoring this patient population. It is vital to keep in mind that desaturation can be a late warning sign of respiratory depression when patients are receiving oxygen. The challenge is that we need respiratory monitoring that can preemptively identify opioid-induced respiratory depression with time to intervene and prevent a critical event. The ideal respiratory monitor should continuously and accurately measure oxygenation, respiratory rate, carbon dioxide tension, and airflow. Take a look at Table 1 from the article and we are going to review it now for a summary of the available respiratory monitoring modalities for detection of opioid-induced respiratory depression.
First up, the mighty pulse oximeter. We are going to pause for a moment so that I can mention that the APSF has dedicated a page to the Pulse Oximetry and the Legacy of Dr. Takuo Aoyagi. We hope that you will check it out to learn all about the history of the invention and innovation of the pulse oximeter. I will include a link in the show notes as well.
Okay, back to the pulse oximeter which measures oxygen saturation. It has the advantages of high availability, well tolerated, and critical threshold values clearly defined. The disadvantages include potentially late indicator of hypopnea and apnea and delayed response times when supplemental oxygen is used. Next up, we have transthoracic impedance plethysmography which monitors respiratory rate. The advantages include high availability, well-tolerated, and age-appropriate critical threshold values clearly defined. The disadvantages are that you may miss hypoventilation due to airway obstruction and there may be motion artifacts with movement.
Our third monitor is capnography which anesthesia professionals use as a routine monitor during anesthesia care to monitor respiratory rate and end-tidal CO2. This monitor is a good indicator of respiratory rate and can provide a close indicator of PaCO2 for intubated patients. The disadvantages include not widely available outside of the operating room and ICU, not well tolerated by children, and inconsistent correlation between end-tidal CO2 and PaCO2 in patients with a natural airway.
Transcutaneous CO2 monitoring provides the skin surface partial pressure of CO2 and has the advantages of good correlation with PaCO2s, and critical threshold values are clearly defined. The disadvantages include limited availability, no breath-to-breath monitoring capability, slow response time which makes it difficult to detect acute changes in ventilation and required calibration every 12 hours or if the monitor becomes dislodged.
Our next monitor is the non-invasive respiratory volume monitor for respiratory rate, tidal volume, and minute ventilation. This monitor is well-tolerated and provides good trending of tidal volume and respiratory rate. Unfortunately, it is rarely available with limited accuracy of minute ventilation measurements in spontaneously breathing patients. In addition, the critical minute ventilation and tidal volume thresholds have not been defined in children.
You have likely seen that continuous pulse oximetry and transthoracic impedance plethysmography are the most common monitors for children. The pulse oximeter made its way onto the scene in the 1980s and provides critical information about oxygenation for infants and children as well as adults. The authors highlight that desaturation may be a late warning sign for respiratory insufficiency or depression, especially when patients receive supplemental oxygen. Pediatric patients may require postoperative supplemental oxygen depending on the complexity of the surgery, patient comorbidities, and/or analgesic requirements to maintain adequate oxygenation. However, this means that there is an increased risk for unrecognized hypoventilation given the increased time between apnea and desaturation.
Transthoracic impedance plethysmography monitors respiratory rate and can identify apnea and hypopnea which is important for patients receiving opioids. We mentioned some of the disadvantages earlier, but keep in mind that the respiratory rate monitoring may be inaccurate due to incorrect ECG electrode placement, motion artifact, and physiologic events that cause chest wall movement (like coughing and crying). Keep in mind that this monitor may not be able to pick up respiratory insufficiency in patients with undiagnosed airway obstruction.
If we are looking for a well-validated monitor of ventilation, we can look no further than arterial PaCo2 levels. But this measurement does not fit our criteria for an ideal monitor since it requires intermittent arterial blood sampling and testing. So, let’s turn our attention to non-invasive surrogate measures of PaCo2 with continuous monitoring of transcutaneous and end-tidal Co2. There are newer transcutaneous PCO2 monitors available that are clinically feasible and safe without the risk of skin burns that were seen in the earlier monitors. There are still some limitations with this monitor since it has not been studied in postoperative pediatric patients receiving opioid medications and has a slow response time. Further technological advances are needed to transform this monitor into an early warning monitor.
Perhaps, we should look no further than capnography for our ideal postoperative monitor than capnography for continuous end-tidal CO2 monitoring that we know provides early and reliable warnings of ventilatory insufficiency for intubated, anesthetized, or sedated patients. If we move outside of the operating room and ICU, capnography with nasal and oral sampling has been studied in adult patients receiving opioids by PCA. In this setting, capnography was found to be a more sensitive indicator of respiratory insufficiency than saturation monitoring. It looks like this may be a good option for an early warning monitor for respiratory insufficiency. In fact, the APSF recommends the use of capnography to monitor ventilation for postoperative patients receiving supplemental oxygen and opioids. There are important limitations with this monitor though since it requires that patients cooperate with wearing the specially designed capnography cannula for long periods of time. The cannulas may be uncomfortable and interfere with activities of eating and talking which decreases patient compliance.
Remember, we are talking about a monitor for pediatric patients and when capnography has been studied in non-intubated, non-sedated postoperative pediatric patients, it was poorly tolerated, and this limits the use of this monitor for pediatric patients. Check out the 2015 study by Miller and colleagues, “Long-term tolerability of capnography and respiratory inductance plethysmography for respiratory monitoring in pediatric patients treated with patient-controlled analgesia.” The average age for the 26 patients was 10 years old and the median time to device removal was 8.343 hours for the capnography. There is a call for the development of more effective, child-friendly, and validated monitors for pediatric patients. I will include the citation in the show notes.
The authors also advocate for clinicians to understand the information provided by capnography. The respiratory rate is accurately monitored, but the end-tidal CO2 values may not reflect the PaO2. Instead, low end-tidal CO2 levels may be an indicator of poor airflow due to unrecognized airway obstruction.
We are excited about newer technologies including non-invasive respiratory monitoring for tidal volume and minute ventilation that have been validated in adults and intubated, ventilated infants and children under general anaesthesia. Tidal volume and respiratory rate trending is good with these monitors, but there is limited accuracy of minute ventilation. These monitors may play an important role for trend monitoring going forward. Check out Figure 3 which depicts preliminary data from 24-hour monitoring of oxygen saturation, respiratory rate, transcutaneous CO2, minute ventilation, tidal volume, actigraphy and PCA opioid use in adolescent patients following posterior spinal fusion surgery. The blue arrows show PCA bolus doses and the following decreased tidal volume. These monitors are likely tolerated in adolescent patients, and it may be feasible to use this monitor for pediatric patients. There is still work to be done since critical ventilatory threshold values to predict or detect respiratory insufficiency have not been clearly defined in children.
The authors leave us with an outlook for the future when we hope to see the use of multiple, complementary monitors with paradigms to include pediatric-specific threshold alarm parameters that will provide earlier identification of episodes of respiratory insufficiency to help keep pediatric patients safe.
Before we wrap up for today, we are going to hear from Monitto again. I also asked her what she hopes to see going forward. Here is her response.
[Monitto] “Throughout my career, I’ve witnessed many advances in anesthesia, critical care, and pain management. Some have come from the development of better medications, and some from better use of our drugs. Other advances have stemmed from improvements in our ability to monitor patients while they’re under our care.
Moving forward, I hope we can improve our patients care even after we’ve left their bedsides. For more UN videos visit www. un. org I believe this will require increased use of continuous monitoring outside the operating room in ICU. In addition to our current monitors, I think we need to develop new patient friendly monitors that can better identify subtle deteriorations in ventilation.
I also hope machine learning algorithms can be developed that will use real time data to more quickly identify worrisome physiologic changes. That will provide us with more opportunities to intervene before serious complications can occur.”
[Bechtel] Thank you so much to Monitto for contributing to the show today.
If you have any questions or comments from today’s show, please email us at [email protected]. Please keep in mind that the information in this show is provided for informational purposes only and does not constitute medical or legal advice. We hope that you will visit APSF.org for detailed information and check out the show notes for links to all the topics we discussed today.
If you have not done so already, we hope that you will rate us and leave a review on iTunes, Spotify, or wherever you get your podcasts and feel free to share this podcast with your friends and colleagues and anyone that you know who is interested in anesthesia patient safety. Plus, you can let us know that you are listening by tagging us @APSForg using the hashtag #APSFpodcast on X.
Until next time, stay vigilant so that no one shall be harmed by anesthesia care.
© 2024, The Anesthesia Patient Safety Foundation