Episode #141 A New Era for PONV Management During Anesthesia Care
March 14, 2023Welcome to the next installment of the Anesthesia Patient Safety podcast hosted by Alli Bechtel. This podcast will be an exciting journey towards improved anesthesia patient safety.
Today, we are heading back into the February 2023 APSF Newsletter to talk about an important consideration for all anesthesia professionals and our patients. This is a topic that may come up during a preoperative interview, something that may alter our anesthesia plan intraoperatively, and a postoperative complication that may delay discharge from the PACU. Our featured article today is “Dopamine-Antagonist Antiemetics in PONV Management: Entering a New Era?” by Connie Chung and Joseph Szokol.
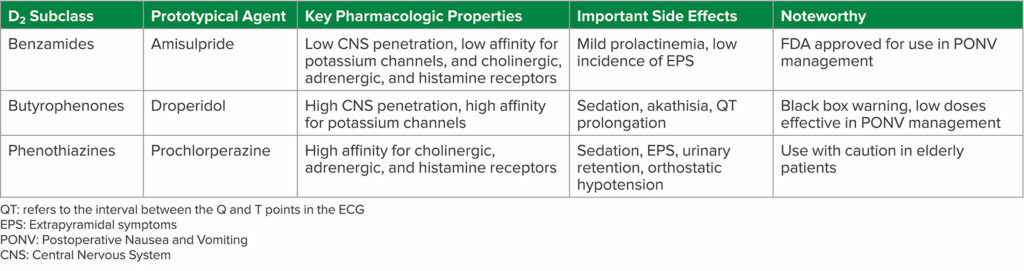
Table 1: D2 Subclass of Antiemetics
Here is the citation for the 2020 Cochrane network meta-analysis that we talked about on the show today:
Weibel S, Rucker G, Eberhart LH, et. al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis. Cochrane Database Syst Rev.2020;10:CD012859. PMID: 33170514.
Be sure to check out the APSF website at https://www.apsf.org/
Make sure that you subscribe to our newsletter at https://www.apsf.org/subscribe/
Follow us on Twitter @APSForg
Questions or Comments? Email me at [email protected].
Thank you to our individual supports https://www.apsf.org/product/donation-individual/
Be a part of our first crowdfunding campaign https://www.apsf.org/product/crowdfunding-donation/
Thank you to our corporate supporters https://www.apsf.org/donate/corporate-and-community-donors/
Additional sound effects from: Zapsplat.
© 2023, The Anesthesia Patient Safety Foundation
Hello and welcome back to the Anesthesia Patient Safety Podcast. My name is Alli Bechtel, and I am your host. Thank you for joining us for another show. Today, we are heading back into the February 2023 APSF Newsletter to talk about an important consideration for all anesthesia professionals and our patients. This is a topic that may come up during a preoperative interview, something that may alter our anesthesia plan intraoperatively, and a postoperative complication that may delay discharge from the PACU.
Before we dive into the episode today, we’d like to recognize GE Healthcare, a major corporate supporter of APSF. GE Healthcare has generously provided unrestricted support to further our vision that “no one shall be harmed by anesthesia care”. Thank you, GE Healthcare – we wouldn’t be able to do all that we do without you!”
Have you figured out what we are talking about today? That’s right, it is postoperative nausea and vomiting. Our featured article today is “Dopamine-Antagonist Antiemetics in PONV Management: Entering a New Era?” by Connie Chung and Joseph Szokol from the February 2023 APSF Newsletter. To follow along with us, head over to APSF.org and click on the Newsletter heading. First one down is the Current Issue. From here, scroll down until you get to our featured article today. I will include a link in the show notes as well. Before we get into the article, we are going to hear from one of the authors, Connie Chung. Let’s take a listen.
[Chung] “Hi, my name is Connie Chung, and I’m an anesthesiologist at the University of Southern California Keck School of Medicine in Los Angeles, California. I’m also the medical director of our outpatient surgery center.”
[Bechtel] To kick off the show today, I asked her why she wrote this article.
[Chung] “Postoperative Nausea and Vomiting, or PONV for short is a topic I’m very passionate about. We want all of our patients to have a great perioperative experience, and PONV can really leave a bad taste in your mouth. All jokes aside, PONV also contributes to prolonged recovery room stays, unanticipated hospital admissions, and increased healthcare costs. Moreover, as a non-smoking female who experiences motion sickness, I know that I personally am in the highest risk category for PONV. It is exciting to see that there is now an FDA approved agent that can be used as rescue treatment of PONV after failed prophylaxis.”
[Bechtel] It is really excited to see a new medication that can be used for treatment of PONV. Before we head into the new era for PONV treatment, let’s review where we have been. The authors remind us that Dopamine D2-receptor antagonists were the primary treatment for post-operative nausea and vomiting for the second half of the last century. Then, at the start of the 21st century, the use of these medications decreased due to safety concerns as well as the publication of a black box warning by the US Food and Drug Administration on Droperidol which was the most commonly used Dopamine D2-receptor antagonists. Have you used Droperidol for the management of PONV in your practice? Did your practice change after the black box warning?
There is a new kid on the block for the treatment of PONV. In 2020, the FDA approved Amisulpride for the prevention and treatment of PONV and it is the only approved agent for rescue treatment after failed prophylaxis.
One of the things we have learned about D2-antagonists is that even drugs that are in this same drug class have different safety and efficacy profiles. There are at least three distinct structural sub-classes with a different pharmacologic properties and side effects. The sub-classes include:
- Substituted Benzamides
- Butyrophenones
- Phenothiazines
Check out Table1 in the article. It includes the subclasses, the prototypical agent, key pharmacologic properties, important side effects, and noteworthy considerations. We are going to review it now.
First up is the Benzamides group. Amisulpride belongs to this subclass. Key properties include the following: Low CNS penetration as well as low affinity for potassium channels and cholinergic, adrenergic, and histamine receptors. The important side effects include mild prolactinemia and low incidence of Extrapyramidal symptoms. Finally, Benzamides are FDA approved for use in PONNV management.
Next up is the Butyrophenones which includes Droperidol. Droperidol has high CNS penetration and a high affinity for potassium channels. Side effects include sedation, akathisia, and QT prolongation. Key considerations include black box warning and low doses are effective in PONV management.
The third subclass is Phenothiazines. Prochlorperazine belongs to this class. Key properties include high affinity for cholinergic, adrenergic, and histamine receptors. Side effects include sedation, extrapyramidal symptoms, urinary retention, and orthostatic hypotension. Administration of this drug to elderly patients may not be appropriate due to these side effects and safety concerns.
Speaking of safety, the authors highlight the safety profile of D2-antagonists. The early D2-antagonists included neuroleptics and first-generation antipsychotics. There was a wide range of neurological effects ranging from sedation to neuropsychiatric including dysphoria or cognitive impairment, due to central nervous system penetration. Another considerable side effects included extrapyramidal symptoms such as tardive dyskinesia, dystonia, and akathisia which limited use. Don’t forget about the risk of neuroleptic malignant syndrome or NMS. Patients may present with fever, mental status changes, muscle rigidity, autonomic instability, and hyperprolactinemia from blockade of D2-receptors in the pituitary gland. There are cardiac side effects as well since binding to potassium ion channels may lead to QT prolongation and torsade de pointes.
The newest medication, Amisulpride, is considered to be atypical or a second-generation antipsychotic with decreased CNS penetration and decreased adverse effects. It is important to keep in mind that some of the side effects from d2-antagonists are dose-dependent, but just decreasing the dose may not be the answer since there is limited data about dose reduction and efficacy. There may be a significant impact on patient safety due to the adverse reactions such as tardive dyskinesia, dysphoria, and torsade de pointes.
Let’s move on to talk about the different classes of medications and we are going to start with Benzamides. Remember, this is the drug class that Amisulpride is in. Amisulpride is a substituted benzamide D2-antagonist and 5-HT2B and 5-HT7A serotonin antagonist with low blood-brain barrier penetration and decreased affinity for adrenergic histamine and cholinergic receptors leading to less anticholinergic and sedative effects. There is also a lower incidence of extrapyramidal symptoms since Amisulpride has preferential binding in the limbic system. There is even more good news in the literature.
A 2020 Cochrane network meta-analysis revealed that Amisulpride has a similar incidence of adverse events when compared to placebo. The risk for adverse effects following administration is lower: Prolactin levels are not elevated above the normal levels for nonpregnant women. Amisulpride also has a weaker affinity for potassium channels and thus does not prolong QT intervals when used at PONV management doses. Finally, amisulpride is effective for preventing PONV as well as for rescue treatment.
Metoclopramide is another medication in this drug sub-class. It is a weak D2 and 5-HT3 antagonist with dose dependent side effects including sedation, extrapyramidal symptoms, and GI upset due to stimulation of gastric smooth muscle cells. Metoclopramide is not as effective for treatment of PONV, but may be considered if there is limited access to other D2-antagonists.
Our next drug class is the Butyrophenones. Droperidol is in this class and historically was administered in low doses for first-line treatment for PONV. Side effects include sedation, dysphoria, anxiety, akathisia, and QT prolongation. You are probably already aware of the risks of QT prolongation since the FDA applied a black box warning in 2001 due to this risk leading to sudden cardiac death. After the black box warning, Droperidol administration for PONV prophylaxis declined significantly. Recently, a new study, the 2020 Cochrane network meta-analysis revealed that antiemetic doses of Droperidol had a similar incidence of adverse events compared to placebo. The most common doses for Droperidol in this study included 0.625-1.25mg IV given at induction of anesthesia. After the FDA black box warning was placed on Droperidol, the focus shifted to Haloperidol, another butyrophenone medication that may be used for treatment of PONV. Adverse side effects included sedation, extrapyramidal symptoms, neurotoxicity, and QT prolongation. In 2007, the FDA updated the labeling for Haloperidol to remind clinicians that this medication is not approved for IV administration for PONV treatment and its use in high doses is associated with torsade’s de pointes and QT prolongation. Keep in mind that a single, low dose of IV Haloperidol may be used for PONV prophylaxis and is likely safe and effective.
The third subclass is Phenothiazines. Prochlorperazine is the most common medication I this class for PONV management. Adverse effects include the following:
- Sedation
- Extrapyramidal symptoms
- Anticholinergic effects such as anorexia, blurred vision, constipation, dry mucosa, and urinary retention
- Antiadrenergic effects leading to orthostatic hypotension
- Decreased seizure threshold.
Another phenothiazine D2-antagonist is promethazine which also has antihistamine effects and may produce sedation. Caution must be used with IV administration since severe tissue damage may occur following extravasation from a vein.
Now that we have reviewed the different subclasses, less talk about side effects from D2 antagonists. Remember, the D2-antagonists are not recommended to be used for patients with prolonged QT syndrome or in combination with other medications that prolong the QT interval. The FDA black box warning on Droperidol highlighted the risk for QT prolongation, but the authors remind us that Ondansetron, which does not have a black box warning and is a commonly used antiemetic medication, can also prolong the QT interval, but the QT prolongation from the combination of ondansetron and Droperidol is not longer than that induced by either drug alone.
There are several home medications that may lead to significant adverse effects when D2-antagonists are administered during the perioperative period.
Be on the lookout for QT prolongation when D2-antagonists are administered for patients taking medications that either reduce the HR or induce hypokalemia.
Patients taking antipsychotic medications are at risk for tardive dyskinesia and NMS.
Do not administer D2-antagonists to patients taking dopamine agonists including Levodopa for Parkinson’s or Cabergoline for hyperprolactinemia.
It is also important to avoid co-administration with monoamine oxidase inhibitors since norepinephrine is broken down by MAO and the D2-blockade leads to build-up of norepinephrine and an exaggerated end-organ response.
One of the APSF Priorities is Perioperative Brain Health which includes perioperative delirium, cognitive dysfunction, and brain health. This is an important consideration with the use of D2 antagonists. The authors advise caution with or avoidance of administration of D2-antagonist for PONV management in patients older than 65 years old due to the risks of central anticholinergic effects (this is the phenothiazine subclass), extrapyramidal symptoms (from the benzamides) and tardive dyskinesia, delirium, and NMS from the butyrophenones. There is an increased risk for cerebrovascular accident, cognitive dysfunction, and mortality in elderly patients who receive these medications. But you must also remain vigilant in pediatric patients who may develop extrapyramidal symptoms or QT prolongation following administration of D2 antagonists.
There is still more to talk about related to D2-antagnoists and PONV management, but we are out of time for today. Tune in next week as we discuss PONV clinical practice guidelines. Plus, we are going to hear from Connie Chung again.
If you have any questions or comments from today’s show, please email us at [email protected]. Please keep in mind that the information in this show is provided for informational purposes only and does not constitute medical or legal advice. We hope that you will visit APSF.org for detailed information and check out the show notes for links to all the topics we discussed today.
If you have not done so already, we hope that you will rate us and leave a review on iTunes or wherever you get your podcasts and feel free to share this podcast with your friends and colleagues and anyone that you know who is interested in anesthesia patient safety. Plus, you can let us know that you are listening by tagging us @APSForg using the hashtag #APSFpodcast and let us know what you use for PONV management in your practice.
Until next time, stay vigilant so that no one shall be harmed by anesthesia care.
© 2023, The Anesthesia Patient Safety Foundation