Introduction
Rather than label aging equipment “obsolete” and removing it from service, health care facilities often place such equipment in a back-up mode — utilized only when patient census and demands are at a peak. While this practice extends the useful life of earlier generations of equipment in the ICU, it may have the unexpected consequence of challenging physicians and ancillary personnel with a dizzying array of unfamiliar equipment.
Case Report
A forty-one-year-old, 90 kg male underwent uneventful aortic valve replacement surgery and was transported to the intensive care unit (ICU) with 100% oxygen via a transport, Mapleson C circuit. Upon arrival in the ICU his blood pressure (BP) was 130/70 mmHg, with epicardial pacing at a rate of 90. He was placed on a Bourns Bear 2®‚ ventilator (Bourns, Inc., Life Systems Division, Riverside, CA) following a standard circuit pressure check of the system. The ventilator settings were IMV = 8 breaths/min, tidal volume of 1 L, FiO2 at 80%, and zero positive-end expiratory pressure (ZEEP).
Within 40 seconds of initiation of mechanical ventilation, acute hypotension developed (BP = 60/40 mmHg). Urgent evaluation by the surgical housestaff focused on a presumed bleeding source or possible cardiac or aortic valve complication — these people were unfamiliar with the Bourns Bear 2®‚ ventilator. Fortunately, arrival and evaluation by an experienced (“older”?) respiratory therapist and attending intensivist noted the needle of the proximal airway pressure gauge was increasing with each breath (without returning to ZEEP during exhalation), as the ventilator was “stacking” each inspiratory breath one upon another.
The patient was immediately removed from the ventilator and hand ventilated with 100% FiO2 via the transport Mapleson circuit. Upon disconnect from the ventilator circuit, the patient’s chest visibly decreased in diameter, with immediate improvement in blood pressure and peripheral perfusion. A follow-up chest x-ray demonstrated no pneumothorax.
The Problem
The ventilator circuit was inspected, and the problem identified as a faulty exhalation valve within the patient manifold assembly. When the valve was disassembled, a 1-cm tear was found in the main body of the valve (see Figure). The exact age of this valve could not be determined, but this particular ventilator had not been utilized in the ICU for more than nine months. The exhalation valve on a Bear 2 tube ventilator is a mushroom shaped, silastic membrane located in the patient manifold within the center of the expiratory limb. As the ventilator cycles to inspiration, a stream of gas is injected into this valve which distends the membrane and closes the expiratory limb of the circuit, thereby forcing inspiratory gas into the patient. During expiration, pressure is released from the valve, allowing the membrane to collapse and opening the circuit to passive exhalation. In our case, the disruption of the valve membrane caused initial expiratory gas in the ventilator circuit to distend the body of the valve, functionally creating an obstruction in the expiratory limb of the circuit. The ventilator therefore “stacked” each inspiratory breath one upon another, resulting in marked air trapping, inhibition of venous return, and severe hypotension. The patient was at high risk for barotrauma and circulatory collapse, but rapid inspection, diagnosis, and appropriate intervention by experienced personnel prevented untoward sequelae.
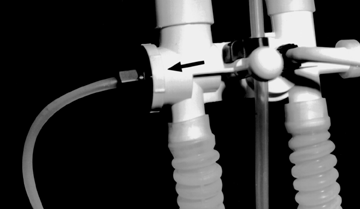
Patient manifold assembly of ventilator with arrow showing location of exhalation valve.

Close-up view of tear in main body of valve membrane.
Discussion
One of only two models of ventilators are currently used within our ICU: the Puritan Bennett 7200 or the Siemens 900. All personnel work with these ventilators daily and are comfortable trouble-shooting problems rapidly. However, the same can not be said for the BEAR 2 ventilator. Most of the surgery housestaff had no experience with this ventilator, which was utilized in this situation because of intense ICU activity and maximal patient census. Thus, the BEAR 2 “back-up” ventilators were urgently and unexpectedly brought back into service in the ICU.
While overt equipment problems are a relatively uncommon source of anesthesia mishaps in the operating room (constituting only 14% of the total number of preventable accidents)2, the situation is quite different for patients in the ICU. Hart reported that equipment problems were the most common source of ICU adverse incidents, most of which affected the cardiovascular or respiratory systems (as with our patient).5 Indeed, Leape found nearly 4% of hospitalized patients sustained disabling injury associated with medical treatment, and that “technical complications” were the third most common type of adverse events (behind drug complications and wound infections). 4 Notably, the ICU was the most common site of care for patients who suffered serious disability (50%), exceeding even the operating room (22%) and the emergency room (25%).4 Fortunately, 42% of events were discovered during routine checks or handovers between shifts of nursing or medical caregivers prior to the occurrence of actual harm to the patient.4
Dr. Gravenstein noted that “obsolete is in the eye of the beholder”.1 Recycling medical equipment may seem very efficient from a financial and accounting perspective, however, we must recognize that use of unfamiliar medical devices contributes to adverse events and errors in patient management. Usually, this applies to utilization of novel or new equipment inserted into service without complete staff training and education. Our case illustrates that (re)use of old equipment may manifest the same limitations and potential for adverse incidents, which applies equally to the operating room and in the ICU.
As noted by Leape, one of the most effective means of reducing errors is standardizing process wherever possible.3 While the BEAR 2 ventilator has been a workhorse in the ICU for many years, its limitations may not be understood by current medical personnel. While the malfunction of the exhalation valve of the BEAR 2 ventilator described here is readily sited by “older” respiratory therapists and ICU personnel, it may be a novel failure to many (or most?) current personnel and trainees in the ICU. Trouble-shooting procedures of the BEAR 2 ventilator must be understood if it is still to be utilized — especially if used only infrequently. Thus, the cost savings of recycling older equipment comes with a hidden price tag attached. That price is the extensive training and education required to maintain expertise and proficiency of personnel on multiple different models of equipment. In our particular situation, the BEAR 2 ventilator is no longer utilized in the ICU. Instead, a lease option has been generated with the medical supplier in the area which provides ICU equipment (including current ventilators) for delivery to the hospital on short notice, and is then returned when no longer needed.
In summary, we must strive to prevent iatrogenic illness and injury, and recognize that the ICU represents a very common location for serious adverse incidents. Unlike the OR, however, equipment failure is a common source of critical events. Proper training and education are required for both new and old equipment — indeed older equipment may represent the greater challenge as earlier models lacked some of the more sophisticated alarms and safety engineering.
Dr. Prielipp and Dr. Morell (who is also Associate Editor of this Newsletter) and Mr. Lewis are from the Department of Anesthesiology, Section on Critical Care, The Wake Forest University School of Medicine, Winston-Salem, NC.
References
1. Cooper JB, Newbower RS, Long CD, McPeek B. Preventable anesthesia mishaps: a study of human factors. Anesthesiology 1978;49:399-406.
2. Hart GK, Baldwin I, Gutteridge G, Ford. J. Adverse incident reporting in intensive care. Anaesth Intens Care 1994; 22:556-561.
3. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. New Engl J Med 1991; 324:377-384.
4. Gravenstein JS. In Response. APSF Newsletter, Summer, 1996.
5. Leape LL. Error in Medicine. JAMA 1994;272:1851-1857.

