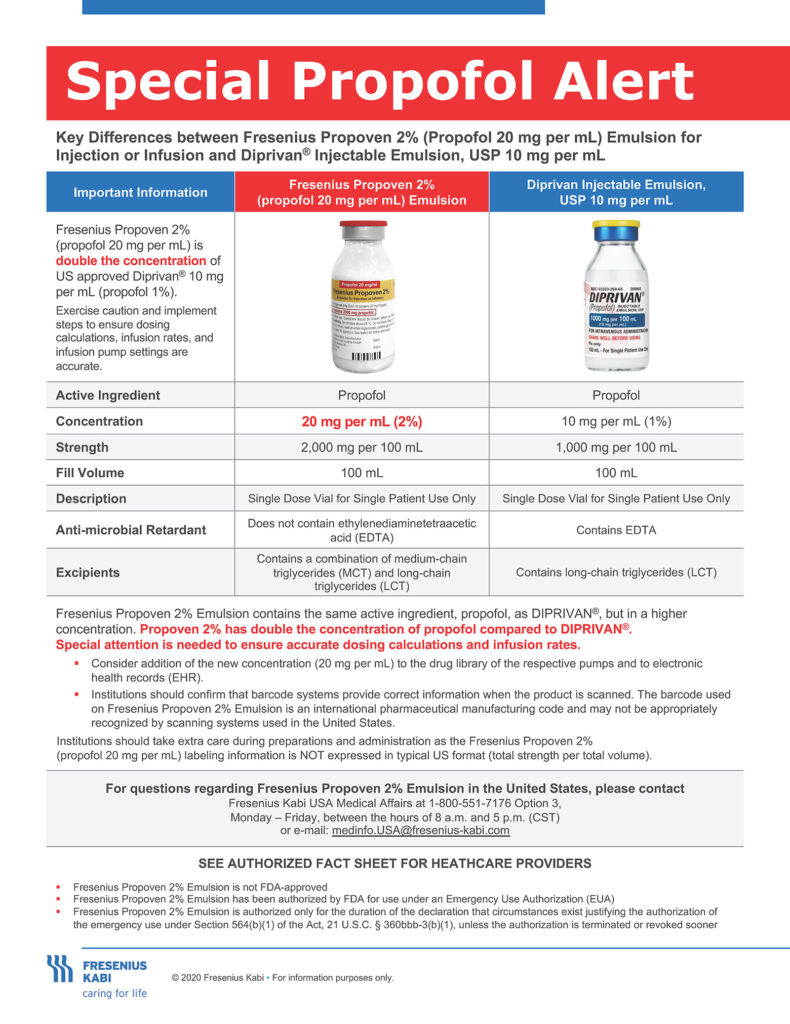
The unanticipated increased use of propofol as a sedative for ventilated patients has strained the nation’s propofol supply for intensive care units. As a result, the FDA has issued an Emergency Use Authorization (EUA) for emergency use of the Fresenius Kabi Propoven 2% Emulsion to maintain sedation via continuous infusion in patients 16 years and older who require mechanical ventilation in an intensive care unit (ICU) during the COVID-19 pandemic. The 2% product will only be available in 100-mL vials, and should only be utilized for long-term continuous infusion in sedated, ventilated patients in an ICU setting. BECAUSE OF THIS DIFFERENCE IN CONCENTRATION BETWEEN THIS NEW 2% (20 mg/mL) PRODUCT AND THE PREVIOUSLY APPROVED 1% (10 mg/mL) PRODUCT, THERE IS A RISK OF UNINTENTIONAL OVERDOSE.
Propoven 2% is expected to be available by mid-June.
Please see the Institute for Safe Medication Practices (ISMP) Special Edition Newsletter for further details.