CASE INTRODUCTION
An 85-year old man without significant medical co-morbidities underwent CT (computerized tomography)-guided biopsy of a peripheral pulmonary nodule. Moderate sedation was administered by an anesthesia professional. The case was uneventful except for mild coughing at the end. The patient was transferred from the CT scanner to a stretcher but the coughing worsened, with the appearance of blood. The anesthesia professional was alarmed and asked CT staff to call for help. There was confusion as to who to call, and in the ensuing minutes, the patient’s hemoptysis worsened. An anesthesia technician arrived with a video laryngoscope, and the anesthesia professional quickly induced general anesthesia and attempted to intubate. The view of the oropharynx was completely obscured by blood. The anesthesia professional performed a cricothyrotomy, which was successful in establishing an airway, but was complicated by hemorrhage from the cricothyrotomy incision. Valiant resuscitative attempts were initiated, but the patient expired within minutes.
This unfortunate case occurred at our institution a year ago. Massive hemoptysis as a complication of interventional radiologic or bronchoscopic procedures is a rare, but catastrophic event that can lead to death within minutes.1 While mortality after massive hemoptysis is high (estimated recently at 13%), having a multi-disciplinary response protocol in place can facilitate life-saving interventions, which may result in improved outcome.1 After this death at our institution, we collaborated with our interventional radiology (IR) colleagues and consulted with the pulmonology, thoracic surgery, and critical care services to develop a massive hemoptysis protocol.
CT LUNG BIOPSY
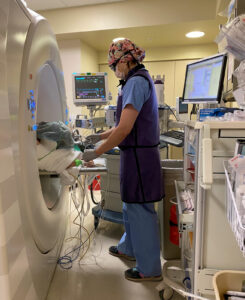
Figure 1. An anesthesiologist working in the CT suite, showing how the CT scanner impedes access to the patient and can make viewing the monitors and ventilator challenging.
Major complications from CT-guided core lung biopsy occur 5.7% of the time, with hemoptysis occurring in 4.1% of core lung biopsy cases according to a 2016 meta-analysis.2 Other adverse events after CT-guided pulmonary biopsy include pneumothorax requiring intervention (manual aspiration or chest tube placement), hemothorax, air embolism, and needle tract seeding.3 Severe hemorrhage with major hemoptysis is the most dreaded and potentially catastrophic complication, occurring 1.8% of the time.4 Risk factors for hemorrhage include patient characteristics such as older age, female sex, coagulopathy, and emphysema. Procedural factors such as larger biopsy needle diameter, increased traversed lung parenchyma and smaller lesion size are also risk factors.2,4,5
Emergent management when hemoptysis occurs is difficult as these procedures are often performed under sedation in the prone position in the small CT suite. General anesthesia with a protected airway could be safer, and should be considered for patients at higher risk for hemoptysis. The challenges of providing anesthesia in non-operating room locations (NORA) were detailed in an article in a recent edition of the APSF Newsletter; many of the concerns raised in that article apply here, including small space, difficult room layout, unfamiliar staff, longer distance from help, older and more medically complex patients (see Figure 1).6
Death from hemoptysis is usually due to blood in the airways preventing adequate oxygenation and ventilation, resulting in patient asphyxiation.1 Historically, there were few rapidly effective treatment options available, and mortality was reported as high as 75%.1 With advancements in airway equipment and prompt intervention including isolating the bleeding lung, patient mortality has decreased to 13% in one study.1 Definitive intervention can be surgical, bronchoscopic (isolation of the bleeding segment with possible balloon tamponade or coagulation) or radiologic (bronchial artery embolization).
PROTOCOL
The Anesthesia Patient Safety Foundation has previously emphasized the need to create protocols to rapidly respond to adverse events.6 We developed a multi-disciplinary protocol to manage a massive hemoptysis emergency. The three main goals of the protocol are
- Secure the airway with a large endotracheal tube (8.0 mm or greater) and isolate the bleeding lung if possible,
- Obtain arterial and venous access for blood pressure monitoring and volume resuscitation,
- Consult specialists for definitive treatment to stop the hemorrhage.1
Secondary goals include positioning the patient with the bleeding side down to help prevent contamination of the other lung until the lungs can be isolated8 and using positive end-expiratory pressure (PEEP) once the airway is secure to tamponade bleeding.7 Once the lungs are isolated, it is preferable to position the patient with bleeding side up to optimize ventilation/perfusion in the ventilating (dependent) lung.
Clear roles are established with anesthesia professionals focused on securing the airway followed by fluid resuscitation. The interventional radiologist is responsible for femoral vascular access followed by consulting specialists for definitive treatment. Possible treatments include bronchoscopic coagulation, interventional radiologic embolization, surgical repair, and/or stabilization and transfer to the ICU. Placement of a chest tube may be indicated based on the location of the hemorrhage. Ancillary staff focus on suctioning blood and calling for help.
We initially developed the massive hemoptysis protocol for interventional radiology-related procedures; however, we also adapted it to apply to hemorrhage associated with bronchoscopies. During bronchoscopy, the rate of major complications is similar to that of interventional radiologic lung procedures, with rates ranging from 0.26% to 5% depending on how complications are defined.7 For bronchoscopy, patient risk factors for pulmonary hemorrhage are malignancy, coagulopathy, and an immunocompromised state.7 Pulmonary hypertension is not a significant predictor of hemorrhage during CT-guided biopsy or bronchoscopy.4,9
The clinical priorities are the same whether hemoptysis occurs in the CT or bronchoscopy suites. However, with endoscopy it may be easier to tamponade bleeding and isolate the bleeding lung from the ventilating lung due to direct visualization by the bronchoscopist. In the CT suite, these cases are often done under sedation; so general anesthesia needs to be quickly induced, but vascular access may be achieved faster with the assistance of interventional radiologists.
TABLE 1. Massive Hemoptysis Protocol for CT/IR
| COMMUNICATION |
|
| PROCEDURE |
|
| ANESTHESIA |
|
| HEMORRHAGE |
|
Abbreviation legend:
CT: Computed tomography
IR: Interventional Radiology
IV: Intravenous
OR: Operating Room
ICU: Intensive Care Unit
Fr: French
LESSONS LEARNED
Our protocol is summarized in Table 1, and we highlight key lessons we learned:
- Getting help in NORA locations is not as straightforward as it is in the OR. Having a clear system to communicate with anesthesia professionals, anesthesia technicians, as well as other specialties that could assist (pulmonology, thoracic surgery, critical care, blood bank) is crucial to getting help quickly. We assign the radiology technologist the task of calling for help. Also, we installed a “staff assist” button next to the code button in the CT suite (see Figure 2) to alert the relevant staff, without having the entire hospital code team show up (as the CT and Endoscopy suites have limited capacity).
- Inducing general anesthesia and securing the airway is of utmost importance and needed clinical care should not be delayed to obtain a CT scan. However, if the hemoptysis is mild and the patient is still in the CT scanner when hemoptysis occurs, a quick CT scan can help visualize the location and extent of bleeding.
- Securing the airway, ideally with one-lung ventilation of the non-bleeding lung, is the highest priority. We assembled a “Hemoptysis toolbox” with supplies to facilitate securing the airway and initiating one-lung ventilation. If the airway is not already secured, early intubation is prioritized, as airway visualization may become impossible as hemoptysis worsens. Given that visualization of the airway may be obscured by blood, we include intubating LMAs for blind intubation and a cricothyrotomy kit.A quick decision must be made to intubate with a large bore single lumen endotracheal tube or a double lumen tube. If bleeding is in the left lung, the single lumen tube can be advanced far enough to provide isolated ventilation of the right lung. If bleeding is in the right lung, lung isolation after intubation with a single lumen tube can be achieved by placement of a bronchial blocker, though a double lumen tube is likely to provide superior lung isolation.7 Direct visualization of bronchial blocker placement may be difficult, while a double lumen tube can be placed blindly after passing the vocal cords. It may be difficult to suction out of the small lumen of the double lumen tube.1,7 Jet ventilation should be considered if isolation of the bleeding lung is not possible, and extracorporeal membrane oxygenation (ECMO) could be considered, if rapid cannulation is possible. However, the patient may require transfer to the operating room for ECMO cannulation given the confined space of the CT scanner room.
- Patient survival may also depend on utilizing the skills of our colleagues from other specialties. The interventional radiologist’s task during the massive hemoptysis emergency is to obtain femoral arterial and venous access. After this, they may place a chest tube if hemothorax is a significant clinical problem associated with pulmonary hemorrhage.Pulmonologists may assist by intubating the non-affected lung via bronchoscopy. If the patient is transferred to the endoscopy suite, pulmonologists could isolate the bleeding segment, instill ice cold saline, tamponade the hemorrhage, and possibly cauterize the bleeding vessel.7
- Once the airway is secure and vascular access obtained, the patient should be positioned with bleeding side down if the lungs are not isolated. This may keep the hemorrhage in the dependent lung to prevent blood contamination of the non-dependent lung.8 If lung isolation is successful, the bleeding side should be positioned up to provide better ventilation/perfusion matching in the dependent lung.
- Extra suction may be needed. CT suites may only have one source of suction. Providing an additional source of suction may be necessary for visualization of the airway and aiding adequate ventilation. One staff member should be assigned to ensuring suctioning equipment is available and working.
- Hemorrhage from hemoptysis is not usually significant enough to require transfusion, but if it is, emergency release blood will be needed as these patients are not routinely typed and screened pre-procedurally. A type and screen should be considered in patients considered particularly high risk for hemoptysis (e.g., women with emphysema or coagulopathy with a more central biopsy target).2,4,5
- Early consultation with other specialties to decide definitive treatment is important. Treatment of the hemorrhage may be surgical, bronchoscopic, or radiologic, but facilitating this discussion and mobilizing the operating room, endoscopy or IR suite is crucial. If the bleeding is self-limited, the patient may be stabilized by the critical care team as the appropriate interventional team is mobilized.
CONCLUSION
Massive hemoptysis is a catastrophic emergency associated with a high mortality rate, but use of a multi-disciplinary protocol and simulations to practice its implementation may improve survival.1 Clear communication and securing the airway as early as possible, isolating the bleeding lung, obtaining vascular access for blood pressure monitoring and volume resuscitation, initially positioning the patient with bleeding side down, and consultation with specialists for definitive treatment of the hemorrhage will maximize the patient’s chances of survival.
Candace Chang, MD, MPH is an assistant professor of Anesthesiology, University of Utah, Salt Lake City, Utah, USA.
Nathaniel Richins, DO is a resident in Anesthesiology, University of Utah, Salt Lake City, Utah, USA.
The authors have no conflicts of interest.
References
- Davidson K, Shojaee S. Managing Massive Hemoptysis. Chest. 2020;157: 77-88.
- Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 2016;27:138–148.
- Lorenz J, Blum M. Complications of Percutaneous Chest Biopsy. Semin Intervent Radiol. 2006;23:188-193
- Tai R, Dunne RM, Trotman-Dickenson B, et al. Frequency and Severity of Pulmonary Hemorrhage in Patients Undergoing Percutaneous CT-guided Transthoracic Lung Biopsy: Single-Institution Experience of 1175 Cases. Radiology. 2016;279:287–296.
- Chassagnon G, Gregory J, Ahmar MA, et al. Risk factors for hemoptysis complicating 17-18 gauge CT-guided transthoracic needle core biopsy: Multivariate analysis of 249 procedures. Diagn Interv Radiol. 2017;23:347-353.
- Walls JD, Weiss MS. Safety in Non-Operating Room Anesthesia (NORA). Anesthesia Patient Safety Foundation Newsletter. 2019;34:3-4, 21. https://www.apsf.org/article/safety-in-non-operating-room-anesthesia-nora/. Accessed Nov. 1, 2020.
- Bernasconi M, Koegelenberg CF, Koutsokera A, et al. Iatrogenic bleeding during flexible bronchoscopy: Risk factors, prophylactic measures and management. ERJ Open Res. 2017;3:00084-2016.
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med. 2000;28:1642-1647.
- Diaz-Guzman E, Vadi S, Minai OA, et al. Safety of diagnostic bronchoscopy in patients with pulmonary hypertension. Respiration. 2009;77: 292–297.
The information provided is for safety-related educational purposes only, and does not constitute medical or legal advice. Individual or group responses are only commentary, provided for purposes of education or discussion, and are neither statements of advice nor the opinions of APSF. It is not the intention of APSF to provide specific medical or legal advice or to endorse any specific views or recommendations in response to the inquiries posted. In no event shall APSF be responsible or liable, directly or indirectly, for any damage or loss caused or alleged to be caused by or in connection with the reliance on any such information.

 Articles
Articles 