Disclaimer: Viewers of this material should review the information contained within it with appropriate medical and legal counsel and make their own determinations as to relevance to their particular practice setting and compliance with state and federal laws and regulations. The APSF has used its best efforts to provide accurate information. However, this material is provided only for informational purposes and does not constitute medical or legal advice. This response also should not be construed as representing APSF endorsement or policy (unless otherwise stated), making clinical recommendations, or substituting for the judgment of a physician and consultation with independent legal counsel.
The ongoing COVID-19 pandemic is prompting consideration of known antiviral drugs for off-label use in the fight against the novel coronavirus. The World Health Organization recently began SOLIDARITY, a program to create worldwide clinical trials in a coordinated effort to collect data during the pandemic. Currently 12 different treatments are being evaluated, including the combination drug lopinavir/ritonavir (LPV/RTV).1 Although a preliminary trial in China of 199 people found no significant difference in time to clinical improvement between patients given lopinavir/ritonavir and those who received standard care, further studies are warranted after lopinavir was found to inhibit the protease activity of coronavirus during the SARS and MERS epidemics.2,3 Additionally, a recent online news article noted the use of LPV/RTV as one component of a 3-drug cocktail for mild COVID-19 disease (https://www.news-medical.net/news/20200512/Antiviral-cocktail-may-help-treat-mild-COVID-19-patients.aspx).
Our purpose is not to provide official comment or policy guidelines on this treatment protocol. It appears that studies and research protocols involving LPV/RTV will almost certainly be ongoing. Clinicians may need to familiarize themselves with this drug co-formulation as well as the numerous, complicated, and clinically relevant drug-drug interactions (DDIs) associated with LPV/RTV in medically complex patients and the information summarized below is provided with that in mind. We feel this information is best approached for the purposes of maximizing perioperative or acute critical care patient safety.
Why Is LPV/RTV Administered as a Drug Combination?
RTV is an oral medication and an HIV protease inhibitor approved by the FDA in 1996 for use in patients with HIV-1. It may be given as a single agent. The package insert for RTV is online at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209512lbl.pdf.
LPV was derived from RTV and approved in 2000. It is also an inhibitor of the HIV-1 protease.
All marketed formulations of LPV are combined with RTV. LPV has an in vitro antiviral EC50 that is ~10-fold lower than that of ritonavir and it is therefore responsible for the antiviral activity of the co-formulation (package insert). However, low dose RTV is used in combination with LPV to inhibit metabolism of LPV in the liver and increase plasma levels and absorption of LPV.4,5 The package insert for the combination drug LPV/RTV is found online at https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021251s052_021906s046lbl.pdf.
Pharmacokinetic Principles for RTV and LPV/RTV
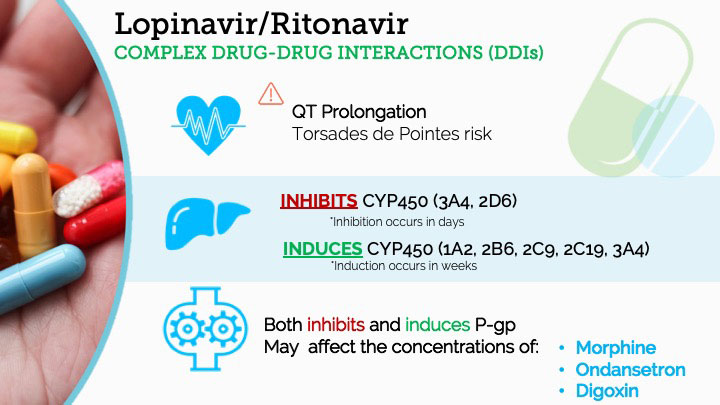
RTV, and to a lesser degree LPV, participate in a complex array of clinically meaningful DDIs involving the cytochrome (CYP) P450 enzyme system. The CYP450 system is a family of metabolic enzymes, found chiefly in hepatocytes, that is largely responsible for Phase I (oxidative) metabolism of a large number of endogenous and exogenous substances. Individual enzyme subtypes have substrates, inhibitors, and inducers. Inhibition of a CYP450 enzyme causes levels of the substrates of that enzyme to increase within a few days. Induction of a CYP450 enzyme will cause increased production or synthesis of the enzyme by the liver over the course of one to four weeks, leading to decreases in substrate levels.
An additional pharmacokinetic variable affected by RTV is the P-glycoprotein (P-gp) pump. This is an extruding ATP-dependent pump found in the gut lumen and the blood-brain-barrier, whose activity decreases bioavailability of its substrates. The P-gp pump also has substrates, inhibitors, and inducers. P-gp function is decreased by inhibitors of the pump, while quantity and thus magnitude of pump function are increased by inducers of the P-gp pump.
Ritonavir DDIs
RTV, when given as a single agent, is involved in a vast array of DDIs. It is also responsible for the significant majority of DDIs that involve the LPV/RTV co-formulation.
RTV is a strong inhibitor of CYP3A4 and a weaker inhibitor of CYP2D6.6 This strong inhibition of CYP3A4 leads to many DDIs that will increase the blood levels of co-administered drugs. The metabolism of several drugs that are of paramount importance to the anesthesiologist will be inhibited by co-administration with RTV, including fentanyl, midazolam, lidocaine, and anti-arrhythmic drugs such as amiodarone. RTV also inhibits the metabolism of other drugs that are commonly administered to medically complex COVID-19 patients, such as rivaroxaban, all systemic steroids, atorvastatin, and immunosuppressant medications (cyclosporine, tacrolimus, etc). Remember, as noted above, this enzymatic inhibition, and the corresponding increases in levels of most other 3A4 substrates, happens within days. Table 1 summarizes a number of other medications that may have increased drug levels when co-administered with RTV.
Table 1.* Ritonavir +/- Lopinavir can inhibit metabolism (increase levels) of the following drugs:
| Drug | Enzyme Involved | Additional Information |
| Antiarrhythmics (amiodarone, bepridil, lidocaine, quinidine) | CYP3A4 | Amiodarone is a pan-inhibitor of CYP450 enzymes |
| Anticancer agents (vincristine, vinblastine, desatinib, nilotinib) | CYP3A4 | Can cause significant hematologic of GI side effects |
| Rivaroxaban | CYP3A4 | Increased risk of bleeding |
| Trazodone | CYP3A4 | Use lower dose to avoid nausea, dizziness, hypotension, and syncope |
| Clarithromycin | CYP3A4 | Must decrease dose only for patients with renal impairment |
| Antifungals (ketoconazole, itraconazole, isavuconazonium) | CYP3A4 | Itraconazole case report, must reduce dose but less interaction than with efavirenz14 |
| Colchicine | CYP3A4, P-gp | Concomitant administration is contraindicated in patients with renal and/or hepatic impairment. Reduce dose and frequency for patients with normal function |
| Quetiapine | CYP3A4, P-gp | Reduce dose to ⅙ of current dose and monitor for quetiapine adverse reactions |
| Midazolam | CYP3A4 | Need close monitoring for respiratory depression and/or prolonged sedation |
| Immunosuppressants (cyclosporine, tacrolimus, sirolimus) | CYP3A4 | Must monitor therapeutic concentration |
| Inhaled or intranasal steroids (fluticasone, budesonide) | CYP3A4 | Can cause reduced serum cortisol concentrations, can lead to Cushing’s syndrome and adrenal suppression |
| Salmeterol | CYP3A4 | Increased risk of CV adverse events associated with Salmeterol including QT prolongation, palpitations, and sinus tachycardia |
| Fentanyl | CYP3A4 | Carefully monitor for potentially fatal respiratory depression |
| PDE5 inhibitors (avanafil, sildenafil, tadalafil, vardenafil) | CYP3A4 | Decrease dose for PAH and ED to avoid hypotension, syncope, visual changes, and prolonged erection |
| Dihydropyridine Calcium Channel Blockers (felodipine, nifedipine, nicardipine) | CYP3A4 | Need dose reduction |
| Rifabutin | CYP3A4 | Reduce dose by at least 75%, decrease frequency |
| Bedaquiline | CYP3A4 | Relatively contraindicated |
| Bosentan | CYP3A4 (and 2C9) | Reduce dose/frequency |
| Simeprevir (Hepatitis C antiviral) | CYP3A4 | |
| Hepatitis C antivirals (ombitasvir/paritaprevir/ ritonavir, dasabuvir | Ombitasvir: CYP2C8
Paritaprevir: CYP3A4 and 3A5 Dasabuvir: CYP2C8 and 3A |
Causes increase in all, however dasabuvir can vary. Not recommended to co-administer with LPV/RTV |
| Systemic corticosteroids (budesonide, dexamethasone, prednisone) | CYP3A4 | Concomitant use increases risk for development of systemic effects, including Cushing’s syndrome and adrenal suppression. |
| HMG-CoA Reductase Inhibitors (atorvastatin, rosuvastatin) | CYP3A4 | Use lowest necessary dose. |
| Indinavir (protease inhibitor) | CYP3A4 | |
| Nelfinavir (protease inhibitor) | CYP2C19 and 3A4 | |
| Saquinavir (protease inhibitor) | CYP3A4 | |
| Maraviroc (CCR5 antagonist) | CYP3A4 | |
| Tenofovir disoproxil fumarate (NRTI) | Cytochrome P450 not involved | Monitor for adverse reactions associated with tenofovir |
** Abbreviations: GI – gastrointestinal; CV- cardiovascular; PDE5 – phosphodiesterase type 5; PAH – pulmonary arterial hypertension; ED – erectile dysfunction; HMG-CoA – 3-hydroxy-3-methylglutaryl-CoA; CCR5 – C-C chemokine receptor type 5; NRTI – nucleoside reverse transcriptase inhibitor
Conversely, RTV is pan-inducer of most of the meaningful CYP450 enzymes. It induces multiple CYP450 enzymes, including CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A4. As such, it may lead to a significant decrease in blood levels of a number of common substances and commonly prescribed medications, including caffeine, oral contraceptives, methadone, and warfarin.7 Table 2 below summarizes these and a number of additional DDIs involving CYP450 enzymatic induction that are noted in the package insert information. Ritonavir also induces glucuronidation enzymes in the liver, specifically the isoform UGT1A4. Thus, drugs metabolized by this system will experience increased metabolism and decreased drug levels, such as lamotrigine.8
Table 2.* Ritonavir +/- Lopinavir can induce metabolism (decrease levels) of the following drugs:
| Drug | Enzyme Involved | Additional Information |
| Caffeine and other xanthines | CYP1A2 | Likely implicates other CYP1A2 substrates |
| Anticonvulsants (lamotrigine, valproate) | Lamotrigine: glucuronidation
Valproate: CYP2C9, 2A6, 2B6 |
Need dose increase |
| Bupropion | CYP2B6 | Monitor for adequate clinical response |
| Voriconazole | CYP2C19 | |
| Atovaquone | CYP2C19 | May need increase in dose |
| Ethinyl estradiol (contraceptive) | CYP3A4 | Less effective as contraceptive, alternative methods recommended |
| Methadone | CYP3A4 | |
| Boceprevir (Hep C antiviral) | CYP3A4/5 | |
| Fosamprenavir/ritonavir (protease inhibitor) | CYP3A4 | Increased rate of adverse reactions |
| Abacavir, Zidovudine (NRTI) | Abacavir: not significantly metabolized by CYP
Zidovudine: hepatic glucuronidation |
|
| Aripiprazole | CYP3A4 | Bipolar relapse, had to increase dose15 |
| Warfarin | CYP2C9 | Decreased INR16
Can see variable levels (Increased or decreased) |
** Abbreviations: NRTI – nucleoside reverse transcriptase inhibitor; INR – International Normalized Ratio
RTV is primarily a CYP3A4 substrate. This means that it is metabolized by CYP3A4 while also being both an inhibitor and inducer of that enzyme. Many other HIV-1 protease inhibitors are also CYP3A4 substrates. Thus, giving RTV with other HIV-1 protease inhibitors allows for lower dosages of either/both to achieve the same biological effects. In general, RTV is more of a “perpetrator” of DDIs than a “victim”, but clinicians should be aware of that certain co-administered medications may increase or decrease RTV blood levels. These are summarized in Table 3 and Table 4 below.
Table 3.* Ritonavir +/- Lopinavir levels can be increased by the following drugs:
| Drug | Enzyme Involved | Additional Information |
| Delavirdine (NNRTI) | CYP3A4 | Increases LPV |
** Abbreviations: NNRTI – Nonnucleoside reverse transcriptase inhibitors
Table 4*. Ritonavir +/- Lopinavir levels can be decreased by the following drugs:
| Drug | Enzyme Involved | Additional Information |
| Anticonvulsants (carbamazepine, phenobarbital, phenytoin) | Carbamazepine: CYP3A4
Phenobarbital: CYP2C9 Phenytoin: CYP2C9, 3A4 |
LPV/RTV is less effective. Can also decrease steady-state phenytoin concentrations |
| Boceprevir (Hep C antiviral) | CYP3A4/5 | |
| Systemic corticosteroids (budesonide, dexamethasone, prednisone) | CYP3A | Decrease of LPV, may be less effective |
| Nelfinavir (protease inhibitor) | CYP2C19 and 3A | Decreases LPV |
| Tipranavir (protease inhibitor) | CYP3A4 | Decreases LPV |
| Efavirenz, nevirapine (NNRTI) | CYP2B6 | Need to increase LPV dose |
| Rifampin | CYP3A4 | Need to increase dose of RTV |
** Abbreviations: NNRTI – Nonnucleoside reverse transcriptase inhibitors
In addition to its various effects on CYP450 and UGT enzymes, RTV is also both an inhibitor and inducer of the P-glycoprotein pump (P-gp). It acts first as an inhibitor and then, later, as an inducer, leading to altered concentrations of P-gp substrates.9 Drugs that are significantly handled by the P-gp include morphine, ondansetron, digoxin, quinidine, carbamazepine, and phenytoin.
Even a cursory review of the tables demonstrates that RTV is characterized by a truly complex array of (sometimes competing) DDI effects. It is of great importance that the prescribing clinician be aware of this. For instance, because RTV is both an inhibitor and inducer of CYP3A4, there may a varied and unpredictable course of DDIs. RTV will initially act as an inhibitor of this enzyme, but in the ensuing weeks after initiation of the drug, the induction profile of RTV at 3A4 can either mitigate, effectively cancel, or even overtake the inhibition effect, producing variable net effects on the levels of various 3A4 substrates (less of an increase, no change, or even a net decrease, respectively).10 A similar phenomenon can occur with regard to P-gp substrates, in light of RTV acting first as an inhibitor and then additionally as an inducer of pump function.
DDIs Resulting from the Lopinavir Component of LPV/RTV
LPV demonstrates mechanism-based CYP3A4 inhibition. LPV is a less potent 3A4 inhibitor than RTV, but still contributes to the net CYP3A4 inhibition that can cause interactions with other drugs with the possible result of serious adverse effects.11 These effects can be due to increased levels of the co-administered medications, increased levels of LPV/RTV, or loss of therapeutic effect and possible development of resistance to LPV/RTV.
Cardiac Considerations of LPV/RTV – QT Prolongation and Torsades de Pointes
Clinicians must be aware that use of LPV/RTV can impact a patient’s cardiac safety by prolonging the QT interval and thus increasing the risk of torsades de pointes.12 A secondary and increased risk of cardiac arrhythmias occurs because inhibition of CYP3A4/2D6 or P-gp by LPV/RTV can elevate blood levels of co-administered drugs that also may prolong QT interval or are otherwise inherently pro-arrhythmogenic. This risk is further compounded by the degree and/or severity to which the clinical course is complicated by co-administered drugs, pathology, and electrolyte imbalances.13
Clinical Context
Why is a thorough review, and investigation of the DDIs associated with LPV/RTV important?
First, clinicians may encounter and/or need to prescribe LPV/RTV in the coronavirus patient cohort during this pandemic. Recall, these drugs are being considered and evaluated for COVID-19 patients as a primary de novo therapy in those who may already have complicated medication regimens. Also, LPV/RTV may be in the standing medication lists for immunocompromised patients who contract COVID-19 disease. Immunocompromised patients have been hit particularly hard by this virus and are in the cohort of people who require novel, therapeutic methods.
Second, the array of LPV/RTV DDIs is unusually complex, with many possible deleterious and significant effects on patient safety. This drug combination interacts with dozens of commonly prescribed medications and these LPV/RTV-associated DDIs can cause an array of clinical sequelae. For example, respiratory depression and/or prolonged sedation can occur in ventilated patients who receive fentanyl infusions or repeated midazolam doses. Also, altered blood levels of potentially toxic drugs with narrow therapeutic windows, such as digoxin, methadone, and warfarin can cause significant problems. And the clinician must always be on the lookout for QT-prolongation and torsades de pointes. To further complicate the issues for clinicians, LPV/RTV has mixed effects on both CYP3A4 and the P-gp, and can act as an inhibitor when first given to a patient but then as an inducer several weeks into the course of drug therapy.
In conclusion, it is not enough to merely identify interacting drugs. Prescribers must remain aware of the sequence and duration of the use of LPV/RTV and co-administered medications to appropriately anticipate and respond to changes in drugs levels, therapeutic efficacy, and clinical events caused by LPV/RTV-associated DDIs. We ourselves review this information with some regularity. We recommend that clinicians who experience confusion, doubt, or uncertainty when caring for patients on RTV or LPV/RTV, either as a contemplated primary therapy for COVID-19 or as a standing medication, should not hesitate to consult with their hospital or critical care pharmacists.
References
- Kupferschmidt K, Cohen J. Race to find COVID-19 treatments accelerates. 2020;367(6485):1412-1413.
- Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):21787-1799. Epub ahead of print, 2020 Mar 18.
- Yao TT, Qian JD, Zhu WY, et al. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J Med Virol. Epub ahead of print, 2020 Feb 27.
- Wyen C, Fuhr U, Frank D, et al. Effect of an antiretroviral regimen containing ritonavir boosted lopinavir on intestinal and hepatic CYP3A, CYP2D6 and P-glycoprotein in HIV-infected patients. Clin Pharmacol Ther. 2008;84(1):75–82.
- Sakuma S, Matsumoto S, Ishizuka N, et al. Enhanced boosting of oral absorption of lopinavir Through electrospray coencapsulation with ritonavir. J Pharm Sci. 2015;104(9):2977-85. Epub ahead of print, 2015 May 18.
- Hsu A, Granneman GR, Bertz RJ. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet. 1998;35(4):275-91.
- Yeh RF, Gaver VE, Patterson KB et al. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy voluntee J Acquir Immune Defic Syndr. 2006;42(1):52-60.
- Van der Lee MJ, Dawood L, Hadewych JM. Lopinavir/ritonavir reduces lamotrigine plasma concentrations in healthy subjects. Clin Pharmacol Ther.2006;80(2):159-68.
- Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother 2008;42(7):1048-59. Epub ahead of print, 2008 Jun 24.
- Fukushima K, Kobuchi S, Mizuhara K. Time-dependent interaction of ritonavir in chronic use: the power balance between inhibition and induction of P-glycoprotein and cytochrome P450 3A. J Pharm Sci. 2013;102(6):2044-2055.
- Weemhoff JL, von Moltke LL, Clemens R. Apparent mechanism-based inhibition of human CYP3A in-vitro by lopinavir. J Pharm Pharmacol. 2003;55(3):381-6.
- Naksuk N, Lazar S, Peeraphatdit TB. Cardiac safety of offlLabel COVID-19 drug therapy: a review and proposed monitoring protocol. Eur Heart J Acute Cardiovasc Care. Epub ahead of print, 2020 May 6.
- Sapp JL, Alqarawi W, MacIntyre CJ et al. Guidance on minimizing risk of drug-induced ventricular arrhythmia during treatment of COVID-19: a statement from the Canadian Heart Rhythm Society. Can J Cardiol. Epub ahead of print, 2020 April 8.
- Hills-Nieminen C, Hughes CA, Houston S et al. Drug-drug interaction between itraconazole and the protease inhibitor lopinavir/ritonavir. Ann Pharmacother. 2009;43(12):2117-20. Epub ahead of print, 2009 Nov 24.
- Hahn M, Roll SC. Dosing recommendations of aripiprazole depot with strong cytochrome P450 3A4 inhibitors: a relapse risk. Drug Safety – Case Reports. 2016; 3(1):5.
- Hughes CA, Freitas A, Miedzinski LJ. Interaction between lopinavir/ritonavir and warfarin. CMAJ. 2007;177(4):357-9.