To meet the exponential increase in demand of SARS-CoV-2 infected patients requiring mechanical ventilation and critical care in New York City, Weill Cornell Medical Center and Columbia University Irving Medical Center expanded intensive care unit (ICU) capacity by converting unutilized operating rooms (OR) into ICUs. We developed a six-step framework and checklist for rapidly and effectively converting ORs to ICUs. These steps include an assessment of appropriate locations, an evaluation of each OR to repurpose and maximize available equipment, coordination with support services to arrange essential critical care infrastructure, and creation of balanced care teams that maximize prior ICU experience. Following this six-step framework and checklist, we increased ICU capacity by at least 112 beds across 38 ORs at two medical centers within a short period (2-7 days). Other facilities can use this pragmatic, systematic approach to guide conversion of ORs into ICUs in a short timeframe and alleviate critical shortages in ICU care.
Introduction
During the initial pandemic surge in 2020, there was an exponential rise in SARS-CoV-2 (COVID-19) cases in the United States epicenter, New York City.1 A significant number of COVID-19 patients developed hypoxemic respiratory failure require mechanical ventilation and care in an intensive care unit (ICU).2 With the demand for intensive care exceeding the available supply, hospitals in many countries required expansion of their ICU capacity.3–5 During this crisis, non-essential surgeries had been postponed, leaving many operating rooms (ORs) and their equipment unutilized.6,7 The United States Food and Drug Administration approved the use of anesthesia machines (AMs), which are used during surgery to deliver respiratory support and anesthetic gases, to be repurposed as ventilators.8,9 As had been reported in Italy, ORs offer one area where hospitals may be able to quickly expand their ICU capacity to address critical shortages.10
To address ICU shortfalls in New York City, New York Presbyterian Hospital’s campuses (Cornell and Columbia) converted ORs into ICUs to support critically ill patients with COVID-19. Based on our experiences, we developed a systematic approach to expanding ICU capacity in the ORs. Here we provide a six-step framework to help hospital leaders evaluate the feasibility of converting their OR’s (or other non-ICU spaces) into ICU’s as well as a checklist of key steps for managing this rapid transition.
Methods
OR to OR-ICU Emergency Conversion Framework
The following six-step framework was devised to help hospitals quickly evaluate the potential for converting ORs and other hospital spaces into ICUs. This framework is adaptable to individual hospitals and should serve as a guide for hospital leaders who seek to optimally use their available infrastructure and resources in order to stay ahead of the demand for critical care. This framework provides a common language for physicians, nurses, administrators, facilities, and support services to work together to rapidly convert ORs into ICUs to treat COVID-19 patients. In our hospital, this was co-managed by a designated surgeon and anesthesia team who were in frequent communication with the institution’s emergency command center leaders, facilities team, and other support services.
Step 1 Determine if this can and should be done at your hospital
The scale of the COVID-19 pandemic has forced hospital leaders to evaluate ICU surge capacity. Any hospital currently offering ICU level care and anticipating a surge of ICU patients in their community should consider converting ORs or other underutilized hospital areas into additional ICU space. Hospitals without ICU capacity, or facilities that do not offer ICU level care, such as endoscopy centers or ambulatory surgery centers, should consider the feasibility of this process on an individual basis as there may be specific challenges at those locations that we do not address here.
Step 2 Identify where it can be done
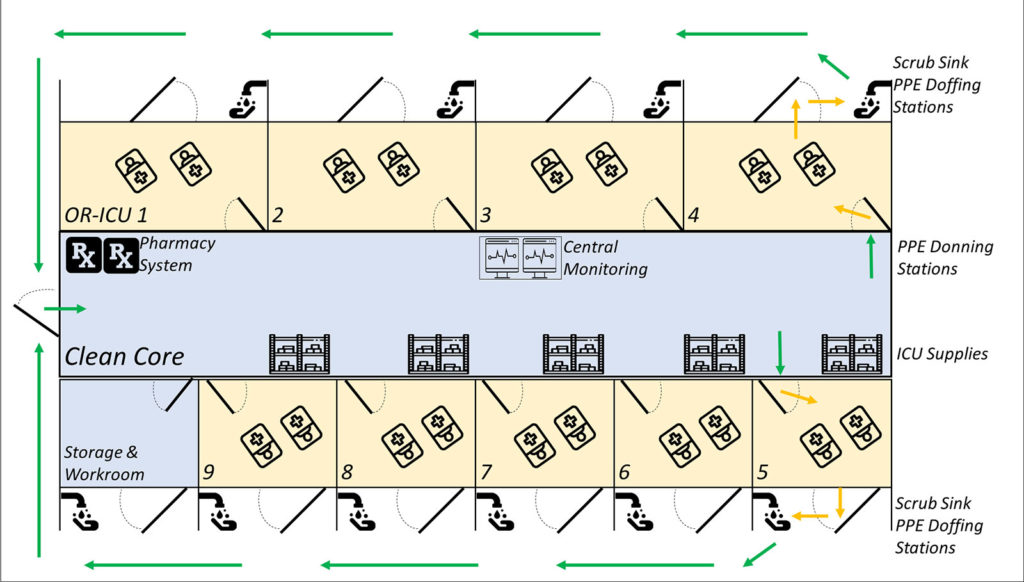
Next consider locations where ICU care can be replicated as this framework can be considered for any hospital space. We identified ORs best suited for ICU conversion based on patient pathway to and from the ORs, safe donning and doffing workflow for personal protective equipment (PPE), a central area for monitoring, supplies, and ventilation systems amenable to becoming negative pressure. In our hospital, ORs are clustered around central cores where surgical supplies are stored. These central cores were converted into nursing stations by installing central monitoring areas, automated pharmacy management systems, and supply racks or cabinets; an example OR-ICU cluster layout and workflow is shown in Figure 1. A subset of ORs were not converted, so emergency surgical services (e.g. obstetric, vascular, and trauma services) could continue.
Although we did not consider creating negative pressure environments to be a critical factor, we prioritized locations where this was possible in order to reduce the risk of viral spread and conserve PPE. In some locations, our facilities and engineering teams were able to adjust ventilation systems and place high-efficiency particulate air (HEPA) filters over air ducts to create negative pressure environments in the OR-ICUs. In other locations, we placed industrial HEPA filters through OR doors to pump air out of the OR-ICUs and create negative pressure environments. These measures decreased contamination of clean areas and improved workflow by allowing providers and support staff to enter the clean cores without additional PPE, only donning and doffing protective gear when entering or exiting the OR-ICUs.
Step 3 Measure how many patients each operating room can accommodate
Next, determine how many patients can be effectively treated in each OR-ICU room. With the aim of maximizing ICU capacity, we planned to cohort multiple patients per room. In our ORs, two primary limitations were spacing, which will depend on the size of anticipated equipment and the number and flow rates of medical gas lines (pressurized oxygen and air) available. In rooms where spaces for patients outnumbered available gas lines, facilities teams should assess the feasibility of splitting these gas lines to support additional anesthesia machines or ventilators. Secondary limitations were the number of dedicated power outlets and data ports in each OR and maximum use was approved by the hospital facilities team.
We planned our OR-ICU capacities based on one patient bed and one anesthesia machine per patient. A surveying team spatially assessed each OR to determine patient configurations that would maximize the number of patients per room while ensuring adequate space between beds for providers and for moving beds and equipment freely.
Patients were cohorted based on COVID positive staus and needs. Although we acknowledge that cohorting actively infected patients is undesirable, we were compelled to create space to treat as many patients as possible. All efforts should be made to reduce cross contamination between patients and to isolate patients who develop secondary transmissible infections (e.g. clostridium difficile colitis).
Step 4 Procure the additional equipment needed to provide ICU level care
Next, identify OR equipment that can be repurposed for ICU care and determine additional equipment needs. Central to our OR-ICU conversion was the repurposing of AMs already in place. Although different in design from standard mechanical ventilators, AMs can serve similar functions.8 Additional AMs were brought into the ORs from spaces that were closed due to the postponement of elective procedures (e.g. endoscopy suites, ambulatory surgery areas, etc.) to increase capacity in our new OR-ICUs. Where additional AMs are not available, these can be substituted with any available standard mechanical ventilators.
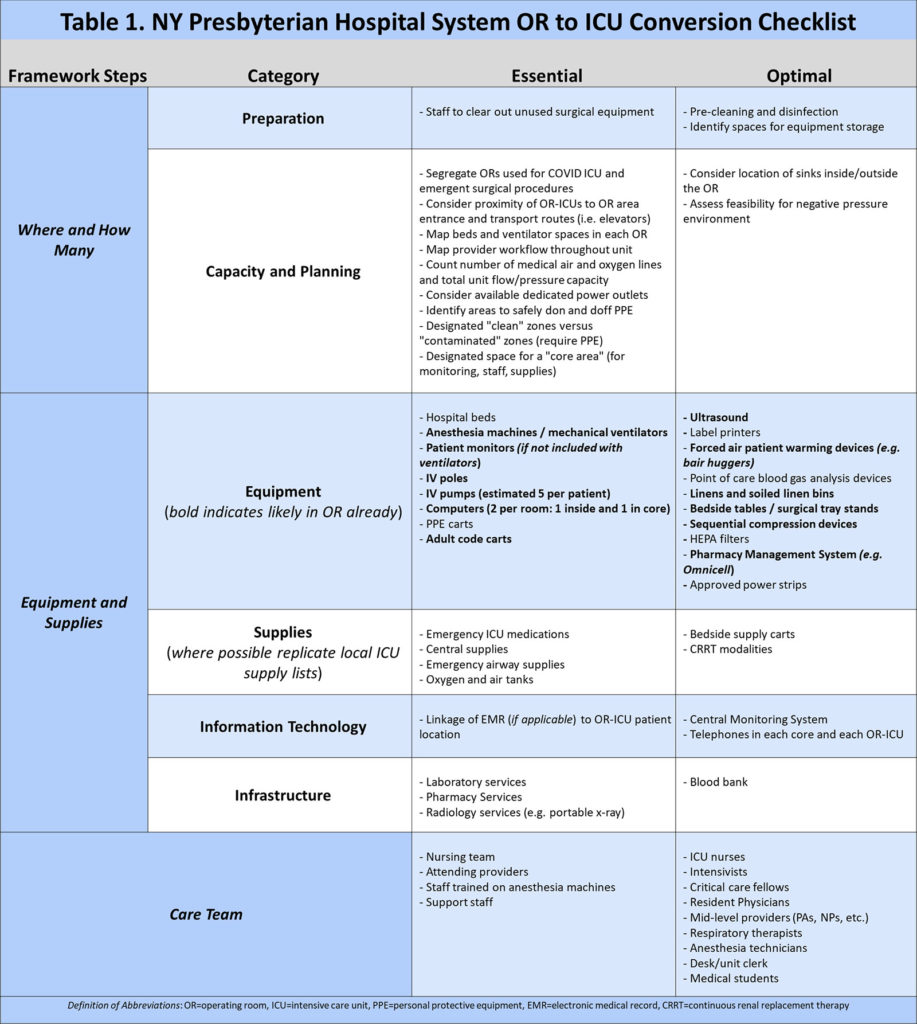
A list of additional essential and optimal ICU equipment (Table 1) was based on standard ICU inventory at our institution. This checklist was determined by input and consensus by ICU leadership, nursing managers, and trainees involved in daily care of COVID patients in traditional ICUs. While comprehensive, this list is not exhaustive and will need to be adapted to local practices and available resources.
In addition, our support services set up the following additional equipment:
- Information Technology (IT) Infrastructure: The information technology and engineering teams revitalized data resources for impending technology and ICU needs. When needed, they added additional data ports in each room.
- Central Monitoring: The biomedical teams set up a station in each core that allowed providers to review vital signs for all OR-ICU patients in that core.
- Supplies: The supply teams created a central supply area in each OR-ICU core and restocked anesthesia supply carts replicating our institution’s standard medical ICU (MICU) supply rooms and bedside cart lists.
- Medications: The pharmacy teams restocked the automated medication management system with standard MICU medications and developed a system for custom medication delivery.
- Communication: The telecommunications teams installed additional phones to facilitate communication between the clean core and OR-ICUs. This reduced the need to open the OR-ICU doors, thus decreasing exposure risk.
- Electronic Medical Records (EMR) Infrastructure: The EMR teams facilitated the creation of a digital OR-ICU unit that clustered these patient records for ease of use.
Step 5 Develop a care team model for the new OR-ICUs
Under surge conditions, it is likely that staffing resources will be overextended at every level. We aimed to recreate the standard care team structure in our institution’s ICUs.11 Available perioperative staff and other non-ICU providers, especially nurses, underwent condensed critical care skills training and ICU preceptorships before being re-deployed into ICU roles. Because we used AMs in our OR-ICUs, we focused on utilizing providers familiar with AMs (e.g. Anesthesiologists and certified nurse anesthetists) instead of respiratory therapists who primarily manage standard ventilators at our institutions. A majority of the certified nurse anesthetists (CRNAs) had previous critical care nursing experience and helped manage the AMs afer brief training sessions which included documentation in the EMR. CRNAs used the ARDSnet ventilatory protocol to guide AM management and changes were discussed with the primary critical care team.12
To address care team shortages due to both quarantined employees as well as the increased patient volume, our staffing structures and provider to patient ratios were frequently modified to balance safety, efficacy, and patient surges. Additionally, some underutilized non-clinical employees were redeployed to assist with other heavily burdened support services, such as supply chain management.
We anticipated there would be changes to the predicted workflow in this unprecedented work environment and stressors for those working in a new capacity. We created a shared online communication platform for those engaged in the OR-ICUs to accelerate quality improvement implementation. For example, based on feedback, we protocolized a system to reconfigure the phones as a two-way intercom to improve communication between providers in the patient room and in the clean core.
Step 6 Consider patient admission
We were able to stay ahead of the demand curve by starting this conversion process before our traditional ICU had reached capacity. We frequently communicated to hospital leaders when beds first became “emergent ready,” indicating they had the minimum necessary life sustaining equipment to care for patients, as well as when they became “optimally ready,” indicating they had been more thoroughly furnished. This allowed us to admit our first patients shortly after the first OR-ICUs were completed, rather than waiting for the entire unit to be operationalized. This approach to preparedness had the added benefit of allowing us to increase patient volume in the new OR-ICU gradually, giving providers time to troubleshoot unanticipated issues before the unit reached capacity. Initially we selected patients from our established ICU’s that were stable enough for transport to be admitted as transfers to the OR-ICU. This allowed the OR-ICU team to acclimate to the new space before admitting the most critical patients.
Results
Following the above framework, we were able to rapidly convert a group of 38 ORs at NYP into novel ICUs with capacity for at least 112 patients respectively. Specifically, at CUMC, we transitioned 23 operating rooms into multi-bed “OR-ICUs,” admitting the first patients within 48 hours and reaching full capacity with 78 individually ventilated patients within 10 days. At WCMC, we transitioned 15 operating rooms into multi-bed OR-ICUs capable of supporting at least 34 individually ventilated patients on anesthesia machines, with additional surge capacity pending the procurement of equipment. The first ICU room was ready within 72 hours, and we admitted the first 4 patients, 5 days after initiating this process. After our initial OR-ICU conversion, we adapted this framework to create additional ICU capacity in other perioperative spaces.
Conclusion
Hospital leaders should evaluate OR to ICU conversion as part of their pandemic surge planning. It is imperative that medical centers use a systematic approach, such as this framework and checklist, to guide conversion of ORs into ICUs in anticipation of increased demand for critical care capacity during the COVID-19 pandemic, and to avoid disorganized ad hoc expansions of non-traditional ICUs. This methodology can also be applied in the assessment and development of ICU capacity in other hospital areas, including post-operative recovery rooms or elective procedure areas, to accommodate additional ICU patients. Rapid conversion of ORs to ICUs is feasible in a short timeframe and can alleviate critical shortages in ICU care.
Acknowledgments
The authors acknowledge the enormous contributions to these efforts by Mary Cassai, Leo Bodden, Dr. Katherine Heilpern, Dr. Laureen Hill, Mollie Kurshan, Lindsay Kanner, Dr. Jonathan Hastie, Dr. Trishia Brentjens, and Hillary Shaw as well as the New York Presbyterian Supply Chain, Facilities, Core Resources, Biomed, Pharmacy, Information Technology, Patient Quality and Safety, and Infection Prevention and Control teams. Their enthusiasm and dedication to patient care undoubtedly resulted in the saving of many lives during the COVID-19 pandemic.
†Designates equal contribution to authorship
Kashmira Chawla† is an Instructor in Anesthesia at Harvard Medical School and staff physician in the Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA
Alex Peters† is a General Surgery resident in the Department of Surgery, Weill Cornell Medical Center/New York-Presbyterian Hospital, New York, NY, USA
Holden K Groves is an Assistant Professor of Anesthesiology at CUMC and staff physician in the Department of Anesthesiology, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA
Keith Denison is a Certified Nurse Anesthestist and Director of Anesthesia Support Services in the Departments of Anesthesiology and Perioperative Services, Weill Cornell Medical Center/New York-Presbyterian Hospital, New York, NY, USA
Natalia S Ivascu is a Professor of Anesthesiology at WCMC and Chief of Critical Care Anesthesiology and the Director of the Cardiothoracic Surgical Intensive Care Unit in the Department of Anesthesiology, Weill Cornell Medical Center/New York-Presbyterian Hospital, New York, NY, USA
David S Wang is an Assistant Professor of Anesthesiology at CUMC and staff physician in the Department of Anesthesiology, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA
Oliver PF Panzer is an Associate Professor of Anesthesiology at CUMC and Critical Care Medicine physician in the Department of Anesthesiology, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA
Zachary A Turnbull is an Assistant Professor of Anesthesiology at WCMC and Medical Director of Perioperative Services in the Department of Anesthesiology, Weill Cornell Medical Center/New York-Presbyterian Hospital, New York, NY, USA
The authors have no conflicts of interest.
References
- Johns Hopkins Center for Systems Science and Engineering. COVID-19 Map – Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed April 7, 2020.
- Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA – J Am Med Assoc. 2020;323:1239-1242. doi:10.1001/jama.2020.2648
- Hostetter M, Klein S. Expanding ICU Capacity in the Midst of a Pandemic: Q&A with Lewis J. Kaplan, M.D., President of the Society of Critical Care Medicine. April 2020. doi:10.26099/BRR8-8K71
- Brito R. The daily terrors: Improvising in a makeshift ICU in Spain – ABC News. ABC News. https://abcnews.go.com/Health/wireStory/daily-terrors-improvising-makeshift-icu-spain-69953680. Published April 3, 2020. Accessed April 7, 2020.
- Krever M, Walsh NP. UK converts convention hall into coronavirus ICU. CNN. https://www.cnn.com/2020/04/01/health/nightingale-hospital-london-coronavirus-intl-gbr/index.html. Published April 1, 2020. Accessed April 7, 2020.
- American College of Surgeons. COVID-19: Recommendations for Management of Elective Surgical Procedures. https://www.facs.org/covid-19/clinical-guidance/elective-surgery. Published March 12, 2020. Accessed April 7, 2020.
- New York State Governor Andrew Cuomo. Executive Order No. 202.10: Continuing Temporary Suspension and Modification of Laws Relating to the Disaster Emergency.; 2020. https://www.governor.ny.gov/news/no-20210-continuing-temporary-suspension-and-modification-laws-relating-disaster-emergency. Accessed April 7, 2020.
- American Society of Anesthesiologists, Anesthesia Patient Safety Foundation. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators.; 2020. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Accessed April 7, 2020.
- U.S. Food and Drug Administration. Ventilator Supply Mitigation Strategies: Letter to Health Care Providers. https://www.fda.gov/medical-devices/letters-health-care-providers/ventilator-supply-mitigation-strategies-letter-health-care-providers. Published March 22, 2020. Accessed April 7, 2020.
- Parodi E, Aloisi S, Barbaglia P. Special Report: “All is well”. In Italy, triage and lies for virus patients. Reuters. https://www.reuters.com/article/us-health-coronavirus-italy-ethics-speci/special-report-all-is-well-in-italy-triage-and-lies-for-virus-patients-idUSKBN2133KG. Published March 16, 2020. Accessed April 7, 2020.
- Kumaraiah D, Yip N, Ivascu N, et al. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM Catal Innov Care Deliv. 2020;10.1056/CAT.20.0158. Published 2020 Apr 28.
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327-336.

 Articles
Articles