Written by the Institute for Safe Medication Practices (ISMP)
ISMP has received reports from several healthcare institutions that recently received cardboard cases labeled “Isoflurane USP 100 mL” by Piramal Critical Care (NDC 66794-017-10) (Figure 1) that actually contained bottles of Isoflurane, USP 100 mL labeled “for animal use only” by Covetrus (NDC 11695-6777-1) (Figure 2). Isoflurane is a general anesthetic administered via inhalation. The products were distributed by at least two different wholesalers, and while all organizations reported the same lot number (H06A22A), we do not know if other lots may be impacted.
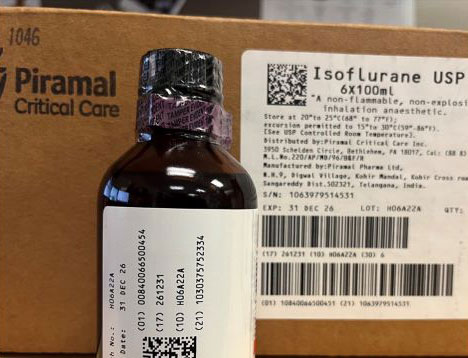
Figure 1. A case labeled Isoflurane USP by Piramal Critical Care contained bottles of Isoflurane, USP 100 mL labeled “for animal use only” by Covetrus.
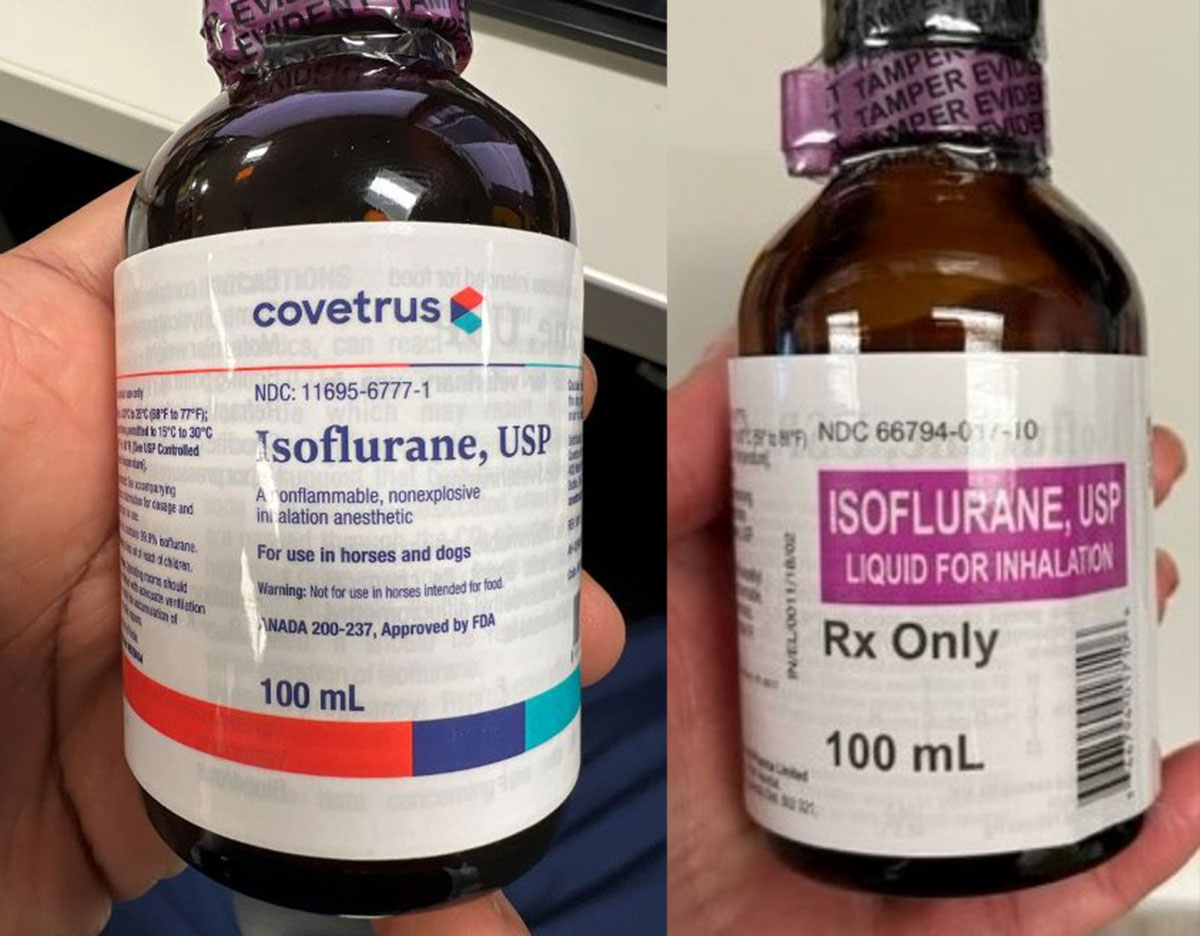
Figure 2. Organizations received bottles labeled Isoflurane, USP 100 mL “for animal use only” (NDC 11695-6777-1) by Covetrus (left) rather than the intended human product, Isoflurane, USP 100 mL (NDC 66794-017-10) by Piramal Critical Care (right).
We have reached out to the manufacturer, Piramal Critical Care, and they are aware of these events and are further investigating. We have also notified the US Food and Drug Administration (FDA). Piramal told us the product labeled “for animal use only” is only approved for horses and dogs and there were no clinical studies completed using this product in humans. They also stated there are no differences in chemical composition between the products labeled for human and animal use. The manufacturer told us that both products are pure isoflurane without any chemical stabilizers and they are made under the same sterile conditions. Even though the products have the exact same composition, if a practitioner receives this product they may be shocked to find it labeled “for animal use only.”
If you purchase this product, inspect your supply to confirm you have the product approved for human use (NDC 66794-017-10) and do not use isoflurane labeled “for animal use only” (NDC 11695-6777-1). Sequester the product and notify the manufacturer, ISMP, and the FDA.
The Institute for Safe Medication Practices (ISMP) is an independent, nonprofit agency dedicated to medication safety. ISMP accepts no advertising in its publications or other work product. ISMP is an affiliate of ECRI, an independent, nonprofit organization improving the safety, quality, and cost‐effectiveness of care across all healthcare settings worldwide. Visit ISMP and ECRI.
Source: https://www.ismp.org/alerts/isoflurane-labeled-animal-use-only-cartons-isoflurane-intended-human-use
