Erroneous Labeling of Phenylephrine Vials Could Contain Cisatracurium in Certain Vials from Meitheal Pharmaceuticals
The Institute for Safe Medication Practices (ISMP) has reported that a potentially extremely hazardous vial packaging error has occurred involving mis-labeled cisatracurium vials from Meitheal Pharmaceuticals.
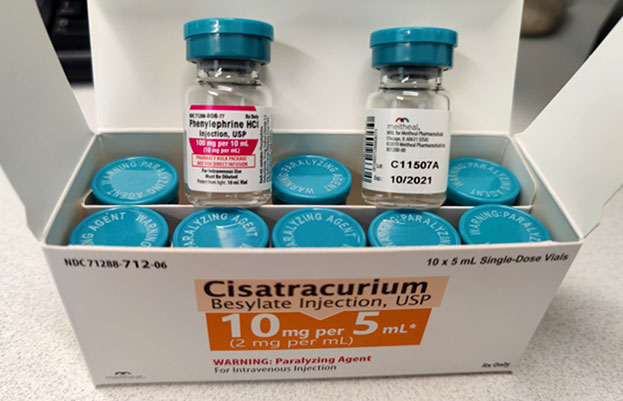
This carton was labeled cisatracurium (correctly) but cisatracurium-containing vials within it labeled “Phenylephrine HCl”. Photo credit: ISMP at https://www.ismp.org/alerts/hazardous-packaging-error, accessed 1.27.2021
As the ISMP notes, the vials have caps that contain appropriate for identifying it as a paralyzing agent. However, the cap may be disregarded or not noticed, since it is afront labeled as Phenylephrine HCl.
A drug error of this nature could have serious potential medical consequences. ISMP recommends facilities to examine any and all cartons of cisatracurium from Meitheal Pharmaceuticals. ISMP has confirmed that the FDA and the manufacturer are aware of this situation and recall is pending and imminent.

