Disclaimer: Viewers of this material should review the information contained within it with appropriate medical and legal counsel and make their own determinations as to relevance to their particular practice setting and compliance with state and federal laws and regulations. The APSF has used its best efforts to provide accurate information. However, this material is provided only for informational purposes and does not constitute medical or legal advice. This response also should not be construed as representing APSF endorsement or policy (unless otherwise stated), making clinical recommendations, or substituting for the judgment of a physician and consultation with independent legal counsel.
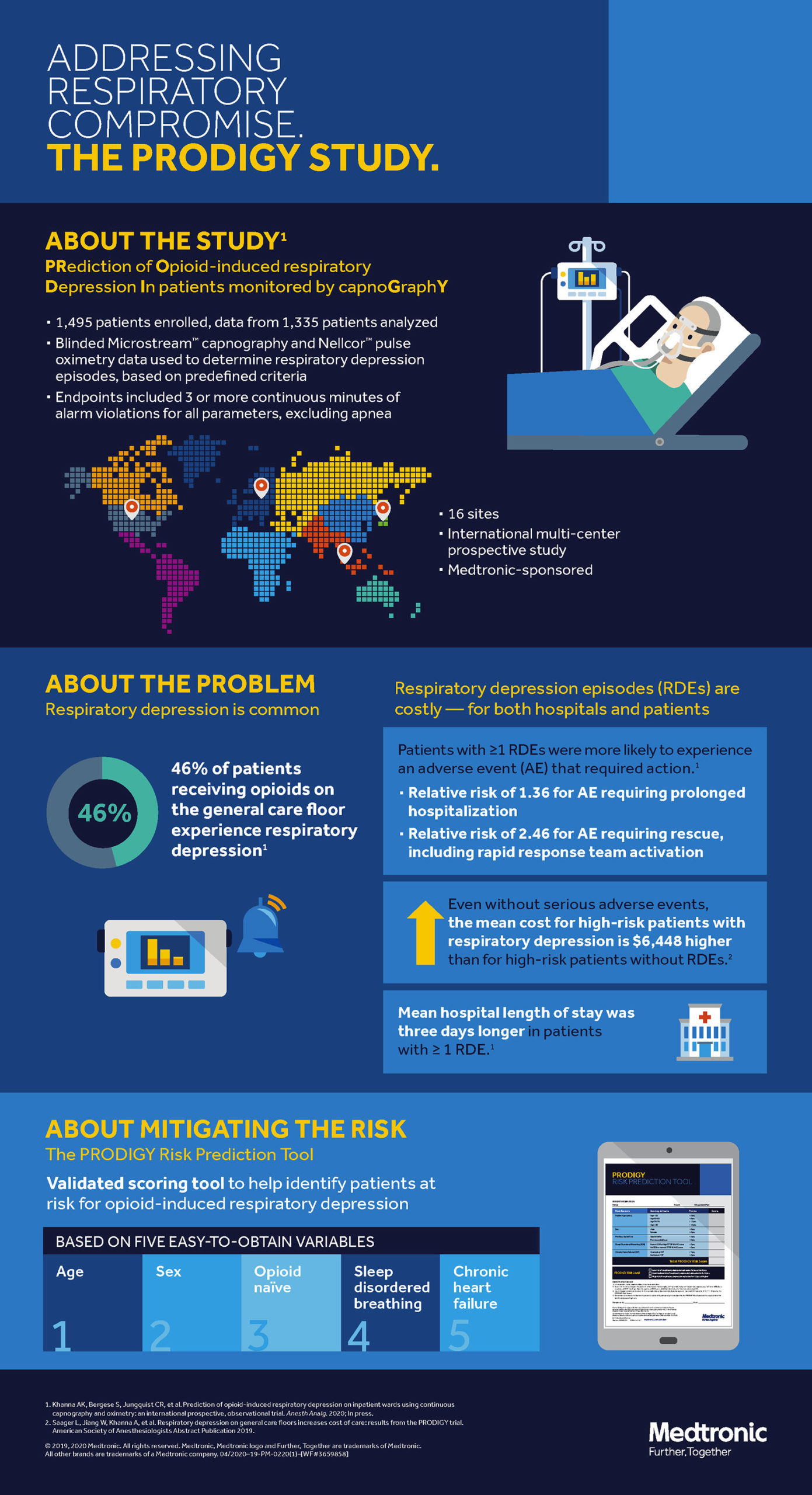
The Anesthesia Patient Safety Foundation (APSF) has been the preeminent organization raising awareness of the dangers of postoperative opioid induced respiratory depression (OIRD), with its first conference on this preventable and often catastrophic risk to patients held in 2006. Following the APSF’s leadership, numerous safety centric organizations such as The Joint Commission, the Centers for Medicare and Medicaid (CMS), Emergency Care Research Institute (ECRI), Institute for Safe Medication Practices (ISMP), and Association for the Advancement of Medical Instrumentation (AAMI) have issued directives and recommendations on when and how to monitor patients on the hospital ward to prevent the tragic outcomes of unrecognized respiratory decompensation. However, despite groundbreaking work on surveillance monitoring, the incidence of OIRD, and contributing factors to this important problem, patients continue to die on hospital wards from unrecognized OIRD.1 To expand the body of evidence for OIRD and improve patient safety, the PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY (PRODIGY) trial was designed to derive and validate a risk prediction tool to allow providers to select patients most likely to benefit from surveillance monitoring with oximetry and capnography.2,3 Across seven countries and three continents, 1,335 patients were continuously monitored using blinded and alarm-silenced capnography and oximetry on the ward while receiving parenteral opioid analgesia. Respiratory depression episodes, defined by traditional thresholds of desaturation, bradypnea, apnea, and hypercarbia were detected in 614 (46%) patients. The PRODIGY score – an easy-to-use risk prediction tool (Table 1) with an area under the curve of 0.74 – was developed using 5 independent variables: age ≥60 (in decades), sex, opioid naivety, sleep disorders, and chronic heart failure.3 The odds for OIRD was 6 times greater in the high PRODIGY risk versus the low risk category (odds ratio 6.07; 95% confidence interval [CI], 4.44-8.30; P<.001). Mean hospital length of stay was 3 days longer in patients with ≥1 respiratory depression episode (10.5 ± 10.8 vs 7.7 ± 7.8 days; P<.0001) and these patients had a higher risk for adverse events requiring rescue (relative risk: 2.46; 95% CI, 1.73–3.50; P<.001).3 Patients with respiratory depression episodes also showed an exponential increase in hospital costs associated with an increased hospital length of stay.
Table 1. The PRODIGY tool, for risk prediction of respiratory depression.3
| Risk Factor | Scoring Criteria | Points Assigned |
| Age (years) | <60 years | 0 |
| 60-69 years | 8 | |
| 70-79 years | 12 | |
| ≥80 years | 16 | |
| Sex | Male | 8 |
| Female | 0 | |
| History of Opioid Use | Opioid Naïve | 3 |
| Previous Opioid Use | 0 | |
| Sleep Disordered Breathing | Known Sleep Disordered Breathing | 5 |
| No Sleep Disordered Breathing | 0 | |
| Chronic Heart Failure | Existing Chronic Heart Failure | 7 |
| No known Chronic Heart Failure | 0 | |
|
Total PRODIGY Score |
Sum of Points Assigned |
|
| PRODIGY Risk Level | Low risk, <8 points total | |
| Moderate risk, 8-14 points total | ||
| High risk, ≥15 points total | ||
The PRODIGY trial supports prior evidence that episodes of respiratory depression defined by traditional thresholds are frequent and undetected.1,4 Fortunately, the number of serious adverse events was low, and this trial verifies that not every episode of desaturation or pause in breathing leads to respiratory arrest. However, the trial suggests that patients who have frequent and prolonged episodes have both worse physiologic and health economic outcomes.5 In fact, patients with monitor-detected episodes had nearly twice the risk of adverse outcomes in the present study. More importantly, expert analysis of the trends of the oximetry and capnography tracings were predictive of patients developing subsequent respiratory distress. These data will form the basis for developing deep learning algorithms from which monitors can more accurately predict patients developing serious OIRD, discarding traditional threshold-based alarms, which contribute to alarm fatigue. Although the bias of being enrolled in a continuous monitoring trial may have contributed to a low number of serious adverse respiratory events, we firmly believe PRODIGY advances the current evidence that continuous surveillance monitoring detects more cardiorespiratory perturbations and may be associated with better clinical outcomes compared with traditional intermittent spot check monitoring.4,6 Outcomes will be critically dependent on the intervention or ‘efferent’ arm, in that appropriate and timely interventions to correct deviations of vitals from the norm will be essential to see a real patient centric benefit.
The next obvious step would be to externally validate this score on an independent cohort, the lack of which was a major limitation of the trial. This would need a robust continuous monitoring data set of floor patients, something that we currently lack, but hopefully will generate once monitoring practices change on hospital wards. Because data collection systems were blinded and alarms silenced, at least a fifth of the collected data was artifactual, though the independent clinical adjudication committee was able to separate these from true episodes. Despite the stated limitations, implementation of the PRODIGY score has significant potential to improve patient safety. While hospitals may not have resources to continuously monitor all patients, clinicians can use the PRODIGY score to evaluate a patient’s risk for respiratory depression, at minimum monitor those at highest risk, and proactively and appropriately intervene at the earliest deviation of vital signs patterns from the physiological norm.
Supplemental Information (From Medtronic®):
- PRODIGY Talking Points: A summary of the study methods, results, and conclusions
- PRODIGY Risk Assessment Tool: Scoring system stratifying risk of respiratory depression based on risk factors such as age, sex, opioid use, sleep disordered breathing and heart failure.
- PRODIGY Fact Sheet: Data and facts behind scoring system, methodology and study design
- PRODIGY FAQ: Frequently asked questions about PRODIGY (PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY)
From the 1Department of Anesthesiology, Section on Critical Care Medicine, Wake Forest School of Medicine, Winston-Salem, North Carolina and Outcomes Research Consortium, Cleveland, Ohio; 2Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and 3Trident Anesthesia Group, LLC, Charleston, South Carolina.
All authors or their institutions report financial support from Medtronic-sponsored PRODIGY trial. In addition, A. K. Khanna reports consulting fees from Medtronic, Edwards Lifesciences, and Philips North America. R.D. Urman reports consulting fees from Medtronic and research funding from Merck.
References
- Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122(3):659-665.
- Khanna AK, Overdyk FJ, Greening C, Di Stefano P, Buhre WF. Respiratory depression in low acuity hospital settings-Seeking answers from the PRODIGY trial. J Crit Care. 2018;47:80-87.
- Khanna AK, Bergese SD, Jungquist CR, et al. Prediction of Opioid-Induced Respiratory Depression on Inpatient Wards Using Continuous Capnography and Oximetry: An International Prospective, Observational Trial. Anesth Analg. 2020;Publish Ahead of Print.
- Sun Z, Sessler DI, Dalton JE, et al. Postoperative Hypoxemia Is Common and Persistent: A Prospective Blinded Observational Study. Anesth Analg. 2015;121(3):709-715.
- Saager L, Jiang W, Khanna AK, et al. Respiratory Depression on General Care Floors Increases Cost of Care: Results from the Prodigy Trial. ANESTHESIOLOGY Annual Meeting. 2019:A2242.
- Turan A, Chang C, Cohen B, et al. Incidence, Severity, and Detection of Blood Pressure Perturbations after Abdominal Surgery: A Prospective Blinded Observational Study. Anesthesiology. 2019;130(4):550-559.

 Articles
Articles