
APSF Technology Education Initiative (TEI) : Low-Flow Anesthesia
One of the barriers to reducing fresh gas flow and the pollution from inhalation anesthetics are those clinical situations where a rapid change in anesthetic concentration is desired. The most rapid change in concentration is achieved when FGF is increased sufficiently to eliminate most, if not all, rebreathing of exhaled gas. In that case, the inspired gas concentration changes to approach or equal the concentration in the fresh gas. How much must FGF be increased to eliminate rebreathing? It is important to estimate this threshold to avoid excessive increases in FGF. Once the objective of eliminating rebreathing is achieved, increasing FGF further creates waste without clinical benefit.
In the APSF course on Low-Flow Anesthesia, it is assumed that when FGF exactly equals MV, rebreathing is zero (a so-called open-circuit condition). While this assumption is useful for simulating and teaching the concepts underlying low-flow anesthesia, the FGF threshold for eliminating rebreathing is not necessarily so exact in real-life—it may be slightly above or slightly below minute ventilation depending upon the workstation. That said, the percentage of exhaled gas rebreathed when FGF approximates minute ventilation is small and not likely to have a significant impact on patient management. The minute ventilation threshold therefore becomes a pragmatic clinical estimate for maximum FGF since it helps to avoid excessive FGF that causes waste and pollution without any clinical benefit. Let’s explore the basis for that recommendation.
It is a generally accepted rule of thumb that once total FGF exceeds minute ventilation, rebreathing will not occur. There are very little data available documenting the FGF threshold that eliminates rebreathing, and no data at all documenting the threshold for circle systems from modern anesthesia workstations.1,2 That said, a thoughtful inspection of the circle system function during inspiration and expiration will help to understand the factors that influence the FGF threshold for eliminating rebreathing.
Eliminating the impact of rebreathing on the inspired anesthetic concentration is desirable when it is time to awaken the patient, but also can be useful during induction or whenever a rapid change in gas and anesthetic concentrations is required. The primary goal in these circumstances is to ensure that the patient’s inspired tidal volume consists almost exclusively of fresh gas. To understand how to achieve that goal, the path of fresh gas flow during exhalation must be understood. During the expiratory phase, fresh gas flows towards the absorbent canister through the rebreathing connection. To eliminate the impact of rebreathing on the next inspiration, enough fresh gas must flow so that the next tidal volume consists of fresh gas only. (Figure 1). For each inspiration, several factors influence whether inspired gas from the rebreathing connection will include previously exhaled gas or not. The most significant factors are total FGF and the internal design of the anesthesia workstation. The internal design, especially the internal volume, will determine how effectively FGF replaces exhaled gases from the rebreathing connection when fresh gas flows in that direction during exhalation. It is interesting to note there are no experimental data to document the influence of these or other factors on the rebreathing threshold.
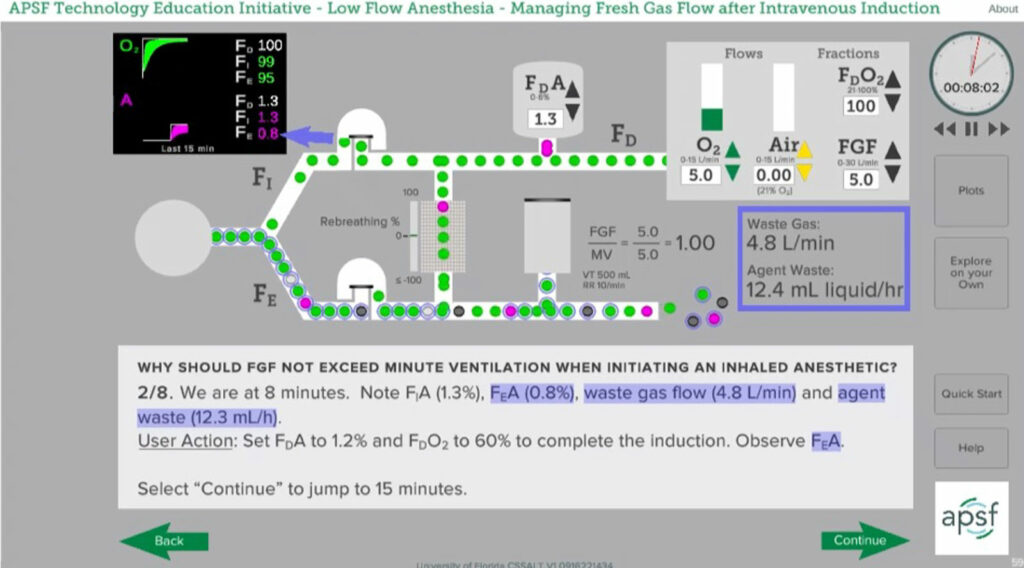
Figure 1: Fresh gas flow equals minute ventilation (5 L/min) and no exhaled gases (indicated by circular border) are being rebreathed. During inspiration gas comes from both the fresh gas inflow as well as the rebreathing connection. Snapshot from APSF Course on Low-Flow Anesthesia. (www.APSF.org/TEI).
Since the various relationships that determine the open circuit threshold for FGF are complex, the clinician can recognize when that threshold has been achieved by using bedside monitors. Here are some clinical methods for determining that FGF is sufficient to minimize or eliminate rebreathing and achieve the desired clinical goal.
Compare the delivered to the inspired oxygen concentration (FDO2 = FIO2): When the inspired oxygen concentration equals or approaches the delivered concentration, the rebreathing percentage is approaching zero. The patient’s oxygen consumption will cause the exhaled oxygen concentration to be lower than the inspired concentration. If there is rebreathing, the exhaled gas will mix with the delivered oxygen and FIO2 will be less than the FDO2. (Figure 1). If FGF is sufficient to prevent rebreathing, the delivered and inspired oxygen concentrations will be the same. (Figure 2)
Figure 2: In this scenario, FGF at 1 L/min is much less than minute ventilation resulting in rebreathing of exhaled gas. FDO2 is 50% whereas FIO2 is 36% due to mixing of exhaled gases with delivered gases. FDO2 is obvious when using a fractional oxygen controller but must be calculated when using oxygen and air flowmeters. Snapshot from APSF Course on Low-Flow Anesthesia. (www.APSF.org/TEI).
It is relatively easy to compare delivered and inspired concentrations when using an anesthesia machine with an oxygen mixer rather than oxygen and air flowmeters. The mixer allows the clinician to set the delivered oxygen concentration and the oxygen monitor on the machine will provide the inspired concentration. Determining FDO2 when using oxygen and air flowmeters requires that the delivered oxygen concentration be calculated from the settings on the flowmeters. The equation to calculate FDO2 from flowmeter settings is simple, but not as convenient as the mixer display.
FDO2 = (O2 Flow + 0.21(Air Flow))/
(O2 Flow + Air Flow)
Inhalation induction: During inhalation induction, the clinical goal is to create a rapid rise in expired agent concentration, commonly called end-tidal agent concentration. The Sevoflurane vaporizer is temporarily turned to maximum, and FGF is increased to deliver the anesthetic, so-called overpressure induction. This is a situation where it is easy to increase FGF well beyond the threshold required to achieve the desired clinical effect causing excessive waste. Monitoring the rate of rise of inspired anesthetic concentration is useful in this case to insure that the desired clinical effect is achieved. If rebreathing is eliminated, the inspired anesthetic concentration should approach the vaporizer setting relatively rapidly that is, FIAgent should approach FDAgent. If there is significant rebreathing, the rate of rise of FIAgent to approach FDAgent is slowed. The rate at which FIAgent rises will depend primarily upon total FGF, but the internal volume of the anesthesia machine and circuit as well as the patient’s tidal volume have an impact. Keeping a good mask seal is of course essential to an efficient inhalation induction.
Carbon dioxide absorbent failure: Let’s assume for a moment that the absorbent in the anesthesia machine became exhausted during a procedure and there is no readily available absorbent supply. When the absorbent is losing effectiveness during low-flow anesthesia, the inspired CO2 will rise. Unchecked, it can become quite high leading to significant hypercarbia that cannot be controlled even by increasing ventilation. A simple solution is to increase FGF until the inspired CO2 is reduced to an acceptable threshold (3–5 mm Hg), or eliminated (0 mmHg).
Eliminating, or nearly eliminating, rebreathing is the primary strategy to maximize the rate of rise of anesthetic concentration in the circuit, and minute ventilation is a useful threshold for the FGF setting required to achieve that goal. Increasing FGF further than MV will increase waste with little if any clinical benefit. That said, when the FGF or vaporizer setting is changed, mixing of fresh gas with the existing gases in the breathing circuit is not immediate. Increasing FGF beyond the threshold of minute ventilation will reduce the time required for mixing. However, the difference in the time required to reach a desired change in anesthetic or oxygen concentrations is quite small as FGF is increased beyond minute ventilation. (Figure 3). The additional waste and pollution is not justified by the marginal increase in rate of rise of FIAgent.
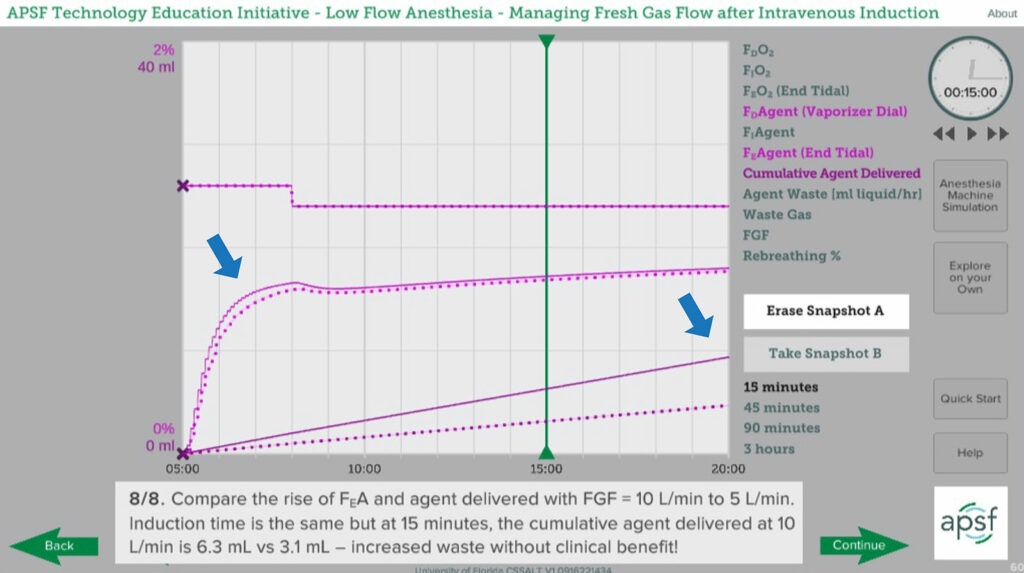
Figure 3: Plots showing rate of rise of exhaled anesthetic concentration with FGF at twice minute ventilation (10 L/min, solid line) compared to minute ventilation (5 L/min, dotted line). Arrows indicate that the rate of rise is only marginally more rapid at 10 L/min in the first few minutes but cumulative anesthetic delivery is much greater. Snapshot from APSF Course on Low-Flow Anesthesia. (www.APSF.org/TEI).
When caring for patients, the bottom line is that the minute ventilation should help to guide the maximum FGF. For adult patients, a maximum FGF of 6 L/min is sufficient and there are few, if any, circumstances that warrant increasing flow beyond that threshold. For smaller adults or pediatric patients, there is an even greater opportunity to reduce FGF since the minute ventilation required is smaller for smaller patients. The Society for Pediatric Anesthesia has published guidelines for weight-based maximum FGF during inhalation induction that can be readily accessed on the society website.3
REFERENCES
- Baum JA. Anesthetic methods with reduced fresh gasflow. In low flow anaesthesia. (2nd ed). Butterworth-Heinemann, Oxford, UK. 2001. P 55.
- Zbinden AM, Feigenwinter P, Hutmacher M. Fresh gas utilization of eight circle systems. Br J Anaesth, 1991;67:492–499. PMID: 1931411.
- Glensky T, Narayanasamy S. Low flow anesthesia in pediatric patients. Society for Pediatric Anesthesia. https://pedsanesthesia.org/wp-content/uploads/2021/08/Low-Flow-Anesthesia-in-Pediatric-Patients.pdf. Accessed October 4, 2022.

 Articles
Articles  PDF
PDF