Contrast agents are frequently administered to patients undergoing computed tomography (CT) and magnetic resonance imaging (MRI) scans as well as patients undergoing chronic pain injections. Patients with shellfish, iodine, or contrast allergy are often pre-medicated. However, this does not necessarily prevent an anaphylactic or anaphylactoid reaction but, would most likely only reduce the severity of the reaction. Prophylaxis medications such as steroids and diphenhydramine can be associated with side effects such as steroid-induced hyperglycemia and diphenhydramine-induced sedation or anticholinergic side effects. Gadolinium, a non-iodinated contrast medium, can serve as an alternative for patients with iodine/contrast allergy. Although comparatively less radiopaque during fluoroscopy, it qualitatively provides comparable images clinically. We present a review of the literature on gadolinium-based contrast agents and the clinical practice of our institutional pain physicians in order to offer clinical insights into its practicality in current interventional pain management in patients with contrast allergy.
Introduction
Contrast reagents are commonly administered in healthcare for studies such as urography, hysterosalpingogram, CT scans, and MRIs. Interventional chronic pain management often utilizes contrast media for procedures as it facilitates proper needle placement and visualization of the spread of medications. Contrast media are helpful for identifying spread within structures including the epidural space, and are helpful for the detection of inadvertent intravascular injection.1 However, providers should be aware and cautious that inadvertent intrathecal administration of specific media such gadolinium-based contrast agent (GBCA) or ionic hyperosmolar contrast agent, can lead to severe or fatal neurotoxic reactions.2 Anesthesia professionals could be requested to provide anesthesia for any of the aforementioned diagnostic tests or interventional procedures. Therefore, anesthesia professionals should have an understanding of the administered medications and possible reactions that can occur in these patients.
A recent study analyzed and compared the relative conspicuity of various gadolinium based contrast agents for pain procedures where Gadobutrol specifically had greatest conspicuity for spinal injections due to this agent having the highest gadolinium molar concentration.3 It also discussed the risks of potential neurotoxicity and discussed digital subtraction being needed for enhancement to facilitate epidural procedures.3 These concerns and modifications may not be necessary for intra-articular injections. Although less radiopaque during fluoroscopy compared to iodinated contrast mediums, GBCA, in our institutional experience, can clinically provide similarly useful images as depicted in Figures 1 and 2.3 A retrospective review of fluoroscopic spot images suggested at least moderate confidence of epidural needle placement utilizing GBCA in 29/38 cases (76.3%) with only 5 cases requiring digital subtraction for image augmentation.4
In this article, we focus on the utility of GBCA, a non-iodinated contrast medium, as an alternative for fluoroscopic-guided non-neuraxial procedures in patients with iodine contrast allergy and briefly review the mechanism of contrast allergy.
Relationship of Iodine and Shellfish Allergy to Contrast Allergy
Patients with shellfish allergy are often identified as having a contrast allergy when iodinated dye is selected as the modality of choice. However, iodine is necessary for normal physiological function.5 Patients with an iodinated contrast allergy are allergic to some component of the contrast medium, but typically not the iodine.6
Atopy confers an increased risk of reaction to contrast administration, but the risk of severe reaction or death is low even in patients with history of iodine allergy, seafood allergy, or prior mild hypersensitivity contrast reaction.1,6 A patient history of prior contrast reaction can increase the risk of mild reactions to as high as 7% to 17%, but has not been shown to increase the risk of severe reactions.6 Patients with an allergy to iodine or shellfish have a 1.5 to 3 fold increased risk of any hypersensitivity reaction to IV contrast agent, and this risk is the same for those who have history of multiple food or drug allergies.1,6 Patients at higher risk for a hypersensitivity reaction to contrast agents include those with a previously documented reaction to the agent which creates a 6-fold increased risk, or a history of asthma with a 5 to 10 fold increased risk.1
Mechanism of Iodinated Contrast Reaction
Symptoms of shellfish allergy range from urticarial to life-threatening anaphylaxis. True anaphylactic allergic reactions are IgE-mediated with rapid onset of symptoms in various organ systems. Management is the same as any other allergic reactions: antihistamines, corticosteroids, and in life-threatening cases, epinephrine.6 On the other hand, iodine has no role in an allergic reaction as it is an integral part of human body and essential for survival, hence not an allergen.1,6 Shellfish allergy is mainly due to specific proteins called tropomyosins.7
As noted previously, reactions to contrast dyes are usually not truly allergic, hence not mediated via IgE, but rather by direct activation of mast cells and basophils to release mediators for an anaphylactoid reaction leading to symptoms similar to anaphylaxis. Patients with allergic reaction to shellfish proteins would create IgE sensitized to the specific allergens. However, these IgE play no role in reactions to contrast media as they are not IgE-mediated.6
The mechanism of anaphylactoid reaction to contrast media is thought to be due to its hyper-osmolality compared to blood. Low osmolality contrast media still uses iodine as a radio-opacification agent but with its lower osmolarity, it has reduced dissociation in solution due to fewer iodinated molecules causing fewer side effects and reactions.8 A case series by Benzon et al., described six patients with a history of hypersensitivity reaction to iodinated contrast medium who were not pre-medicated and had unintentional injection of iodinated contrast during interventional pain procedures. None of these patients developed a moderate or severe reaction.9
Institutional Protocols for Contrast Allergy
Providers often administer institution-dependent protocols of pre-medications for patients listed as having contrast-allergy, shellfish allergy, or iodine allergy. Premedication does not prevent an anaphylactic or anaphylactoid reaction, but helps reduce the severity of the reaction.10 Combinations of corticosteroids and H1-blockers with or without H2-blockers are employed. The protocol (see table 1) employed at our institution (Allegheny Health Network, Pittsburgh, Pennsylvania) is oral prednisone 50 mg Q6 hours (13 hours, 7 hours, and 1 hour prior to contrast administration) for 3 doses and oral diphenhydramine 50 mg (1 hour prior to contrast administration). Alternatives for patients unable to take oral medications include intravenous hydrocortisone 200mg and intravenous diphenhydramine 50mg. Administering the lowest dose and concentration of the contrast while limiting the number of contrast injections should be done to minimize adverse reactions.10
Table 1: (Radiology PreMed Contrast Allergy Order Set) in Allegheny Health Network
| Oral Medications | Instructions |
| Prednisone Tablet | 50mg, oral, every 6 hours, for 3 doses. Pre-procedure give 13 hours, 7 hours, and 1 hour before contrast medium injection |
| Diphenhydramine capsule | 50mg, oral, once, starting at 12 hours for 1 dose, pre-procedure
Give 1 hour before contrast medium injection |
| Injectable Medications | Instructions |
| Hydrocortisone sodium succinate injection | 200mg, intravenous, every 6 hours, for 3 doses pre-procedure. Give 13 hours, 7 hours, and 1 hour before contrast medium injection. |
| Diphenhydramine injection | 50mg, intravenous, once starting at 12 hours for pre-procedure
Then, give 1 hour before contrast medium injection |
Pre-medications reduce the incidence of respiratory symptoms due to contrast media from 1.4% to 0.4% and incidence of combined respiratory and hemodynamic symptoms from 0.9 to 0.2%. Therefore, a significant number of patients need to receive steroids prophylactically to reduce an adverse event.11
Detrimental Effects of Contrast Allergy Pre-Medications
Administration of glucocorticoids can be detrimental to diabetic and pre-diabetic patients by triggering hyperglycemia due to steroid burden.12 Oral steroid therapy (based on treatment of asthma or skin conditions) is associated with an odds ratio of 1.36 for developing new-onset diabetes, as demonstrated in large case-control series.13 Furthermore, steroid use in elderly individuals has been shown to increase the risk of new-onset diabetes with an odds ratio of 2.31 compared with those not receiving steroids.14 Accounting individual variability, both intra-articular and epidural steroid injections can have systemic effects and complications associated with their use. Stout et al. authored a narrative review, which suggested that concurrent use of oral steroids, the number of injections, and the type and dose of glucocorticoids used are all important considerations in estimating risk including but not limited to hyperglycemia, loss of bone density, infection, or Cushing Syndrome.15 Prescribing glucocorticoids for a theoretical allergy should not be routine and should be considered carefully. Unwanted sedation and anticholinergic symptoms from high doses of H1-blockers (commonly diphenhydramine) could possibly prevent the timely meeting of discharge criteria from the clinic.16
Alternative to Iodine Contrast: Gadolinium-Based Contrast Agent
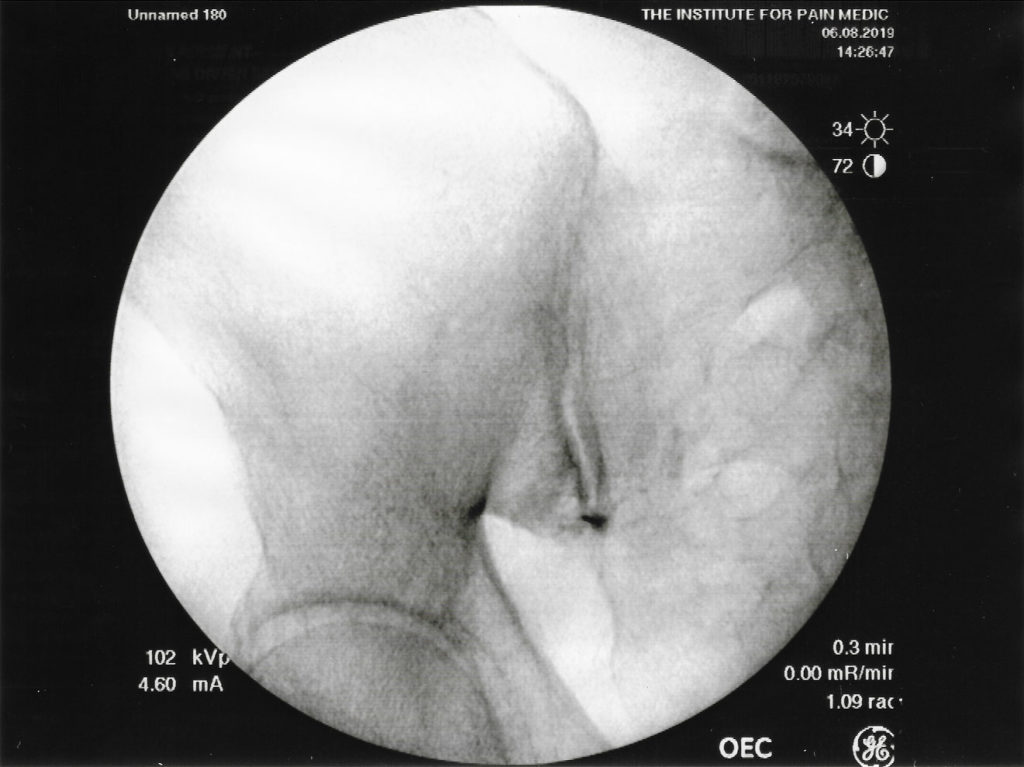
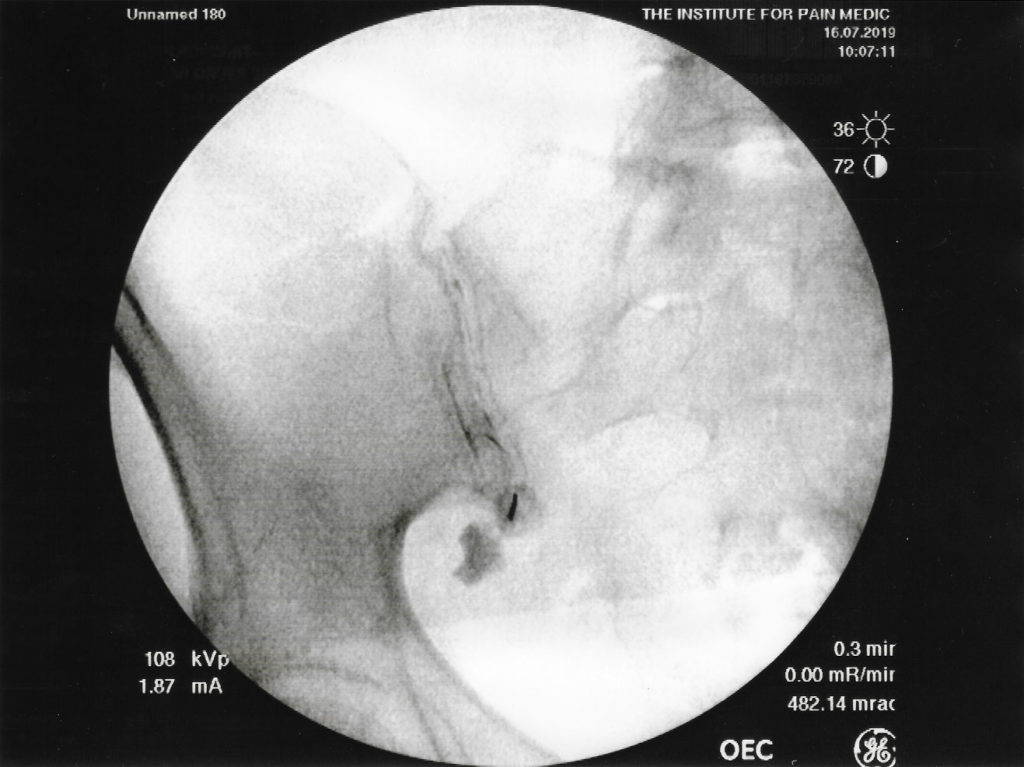
GBCAs have been used as an alternative for fluoroscopic guided procedures in patients with “iodine contrast allergy.” The adverse reaction rate is 0.06% with severe, life-threatening allergic reactions to intravenous gadolinium and a frequency rate of 0.0003% to 0.01%.1 GBCA contains gadolinium, a heavy metal which attenuates X-rays but to a lesser degree than do iodinated contrast agents which attenuate X-rays beams due to their concentrated iodine content. Fluoroscopic images of sacroiliac joint injections at our pain clinic demonstrate how a gadolinium-based contrast agent as seen in figure 1 is less radiopaque compared to an iodinated-contrast agent as seen in figure 2. As the images show, the differences in image quality between GBCA and iodinated contrast; however, appear to be minimal and make the use of an GBCA a clinically useful alternative for fluoroscopic guided procedures such as joint injections. 3
Inadvertent intrathecal GBCA can result in significant neurological consequences including encephalitis, chemical meningitis, and seizures with optic nerve involvement.17 Gadolinium acute neurotoxicity presents within 1 hour of administration where patients experience mental status changes, cognitive decline, involuntary movement, and seizure activities.18 If only a small amount of contrast agent (0.2 to 0.5mL, not exceeding 1mL) is used as typically done for most interventional procedures, the risks of any significant renal (e.g. nephrogenic systemic fibrosis) and neurotoxicity is very low.17,18 A systemic review and meta-analysis indicated intrathecal administration of GBCA at doses greater than 1.0 mmol can be associated with serious neurotoxic complications.17,18 Nephrogenic systemic fibrosis is associated with larger doses of GBCA (>0.2mL/kg) and chronic kidney disease stage 4 or 5.20, 21
A retrospective record search of percutaneous outpatient spinal intervention using GBCA reviewed 92 patients who underwent 127 procedures and found no complications reported.22 All procedures were done successfully with GBCA with no indication of failure due to image compromise. The mild to moderate side effects (nausea, vomiting, or headache) occurred in less than 1% of patients when gadolinium was administered intravenously.22
While contrast is important in confirming proper needle placement to help avoid inadvertent administration of steroids and local anesthetic to areas other than where it is intended, it is crucial to note that GBCA should not be used for neuraxial procedures in our medical opinion, since it is not FDA approved and doing so would have risk of neurotoxicity.
Conclusion
Our institutional practices have resulted in satisfactory image quality when using GBCA as an alternative for those patients allergic to iodinated-contrast when performing non-neuraxial procedures effectively and safely. As expected, GBCAs (figure 1) provide less radiopaque fluoroscopic images during sacroiliac joint injection, compared to iodine contrast (figure 2). Clinically, the use of GBCA allowed successful completion of the procedures with no complaints from our physicians with regards to image quality. As to date, no contrast reactions have been reported at our institution with well over 250 administrations of gadolinium contrast.
Clinical judgment is important in deciding whether or not patients need premedication by evaluating the severity of prior iodinated contrast reactions, occurrence, and amount of contrast administered. The risks of administering steroids and diphenhydramine, especially to susceptible patient populations (diabetics, elderly) should play a role in the clinician’s decision to use iodinated contrast. We recommend consideration of a GBCA as a relatively safe alternative, especially since pre-medications do not necessarily prevent a contrast reaction. There is however the caveat that GBCA can lessen image quality by being less radiopaque. Healthcare professionals that perform these procedures should be aware of the adverse consequences of improper use of GBCA- intrathecal administration leading to neurotoxicity or incorrect dosing leading to nephrotoxicity. Therefore, a discussion with the patient regarding the risk-benefit ratio of the intervention and the potential side effects of contrast agents as well as pre-medications is prudent.
Safety of the patients should be emphasized with preparation with pre-procedural intravenous access and availability of resuscitation equipment if indicated. Using the least amount of dye necessary for completion as well as keeping in mind the risks of GBCA is paramount. Safe practice guidelines should be implemented in practice. We believe that it is reasonable to use GBCA, especially for procedures with no or low risk of inadvertent dura violation such as sacroiliac intra-articular joint injections. Likewise, knowledge of medications given for patients with contrast allergy and possible reactions to these medications and contrast agents will help ensure the safe and appropriate management of patients.
Raymond C. Yu, MD is an anesthesiology resident (CA-3) at the Allegheny Health Network Medical Education Consortium in Pittsburgh, PA. He is the chief resident for scholarly affairs.
Asli Ozcan, DO is an anesthesiology resident (CA-2) at the Allegheny Health Network Medical Education Consortium in Pittsburgh, PA.
Till Conermann, MD is ABMS board certified in Anesthesiology and Pain Management and ABAM/ABMS board certified in Addiction Medicine. He is currently the Program Director for Pain Management Fellowship in the Allegheny Health Network Medical Education Consortium (WPH) program and associate clinical professor of anesthesiology for Temple University. He was the President of the Pennsylvania Pain Society from 2009-2014.
The authors have no conflicts of interest.
References
- Newmark JL, Mehra A, Singla AK. Radiocontrast Media Allergic Reactions and Interventional Pain Practice- A Review. Pain Physician 2012; 15:E665-E675.
- Liu H, Tariq R, Liu L, Yan H, Kaye AD. Inadvertent Intrathecal Injections and Best Practice Management. Acta Anaesthesiol Scand 2017; 61: 11-22.
- Maus TP, Schueler BA, Magnuson DJ, Magnuson D. Relative Conspicuity of Gadolinum-Based Contrast Agents in Interventional Procedures. Pain Med 2017; 18: 651-654.
- Shetty SK, Nelson EN, Lawrimore TM, Palmer WE. Use of Gadolinium Chelate to Confirm Epidural Needle Placement in Patients with an Iodinated Contrast Reaction. Skeletal Radiol 2007; 36: 301-307.
- Zimmermann MB. Iodine requirements and the risks and benefits of correcting iodine deficiency in populations. J Trace Elem Med Biol. 2008;22:81-92. doi: 10.1016/j.jtemb.2008.03.001. Epub 2008 May 7. PMID: 18565420.
- Schabelman E, Witting M., The Relationship of Radiocontrast, Iodine, and Seafood Allergies: A Medical Myth Exposed; J Emerg Med 2010; 39: 701-707.
- Coakley FV, Panicek DM. Iodine allergy: An oyster without a pearl? Am J Roentgenol 1997; 169:951-952.
- Baig M, Farag A, Sajid J, Potluri R, Irwin RB, Khalid HMI. Shellfish allergy and relation to iodinated contrast media: United Kingdom survey. World J Cardiol 2014; 6: 107-111.
- Benzon HT, Schechtman J, Zheng SC, Katz JA, Patel A, Nagpal G, et al. Patients with a history of hypersensitivity reaction to iodinated contrast medium and given iodinated contrast during an interventional pain procedure. Reg Anesth Pain Med 2019; 44: 118-121.
- 10 Ares JD, Amatriain GR, Iglesias CN, Forner MB, Gay MLF. Contrast agents used in interventional pain: Management, complications, and troubleshooting. Tech Reg Anesth Pain Manag 2014;18: 65-75.
- Tramer MR, von Elm E, Loubeyre P, Hauser C. Pharmacological prevention of serious anaphylactic reactions due to iodinated contrast media: systematic review. BMJ 2006; 333: 675 [PMID: 16880193 DOI: 10.1136/bmj.38905.634123AE]
- Hwang, JL, Weiss, RE. Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment. Diabetes Metab Res Rev 2014;30:96-102.
- Gulliford, MC, Charlton, J, Latinovic, R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care 2006;29:2728-2729.
- Blackburn D, Hux J, Mamdani, M. Quantification of the risk of corticosteroid-induced diabetes mellitus among the elderly. J Gen Intern Med 2002;17:717-720.
- Stout A, Friedly J, Standaert CJ. Systemic Absorption and Side Effects of Locally Injected Glucocorticoids. PM R. 2019 Apr;11:409-419. doi: 10.1002/pmrj.12042.
- Huynh DA, Abbas M, Dabaja A. Diphenhydramine Toxicity. 2021 Feb 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 32491510.
- Durbahakula S, Cohen SP. Gadolinium use for interventional pain procedures: where we are and where we are heading. Reg Anesth Pain Med 2019 Jan; 44: 4-6.
- Provenzano DA, Pellis Z, DeRiggi L. Fatal gadolinium-induced encephalopathy following accidental intrathecal administration: a case report and comprehensive evidence-based review. Reg Anesth Pain Med 2019; 44: 721-729.
- Patel M, Atyani A, Salameh JP, McInnes M, Chakraborty S. Safety of Intrathecal Administration of Gadolinium-based Contrast Agents: A Systemic Review and Meta-Analysis. Radiology 2020; 00:1-9. doi: 10.1148/radiol.2020191373. Online ahead of print.
- Popescu A, Patel J, McCormick ZL, Maus TP, Rodes M, Walega DR, Smith CC. Fact Finders for Patient Safety: Are Gadolinium-Based Contrast Media Safe Alternatives to Iodinated Contrast Agents for the Safe Performance of Spinal Injection Procedures? Pain Med 2018. 19:2089-2090.
- Beckett KR, Moriarity AK, Langer JM. Safe Use of Contrast Media: What Radiologist Needs to Know. Radiographics 2015; 35: 1738-1750.
- Safriel Y, Ali M, Hayt M, Ang R. Gadolinium Use in Spine Procedures for Patients with Allergy to Iodinated Contrast – Experience of 127 Procedures. Am J Neuroradiol 2006; 27:1194-1197.

 Articles
Articles