Syringe Labeling Made Simple
Benjamin O. Pate, BS;Bruce P. Kingsley, MD
To the Editor
Syringe swap is one of the most common causes of medication error in the practice of anesthesia.1
These errors are rarely published since they are either of little consequence, are professionally uncomfortable to admit, or result in litigation that inhibits outcome reporting. We are aware of several cases that fit this description. Ephedrine and cefazolin injected into the epidural space were cases that had no consequence. Vecuronium mixed and immediately injected intravenously by an exhausted anesthesiologist into a conscious patient resulted in harm. The intent was to give cefazolin. Thirty-four milligrams of epinephrine in an unlabeled syringe left on the prep table was mistaken for bupivacaine and was injected into the knee of a patient under general anesthesia. Myocardial infarction, pulmonary edema, and cardiopulmonary failure resulted. A properly labeled syringe of mivicurium was injected in error into the caudal epidural space of a young patient by an anesthesiologist who misread the label, hand written by another anesthesiologist as “Marcaine.” Neuraxial injury was purported to be a consequence.

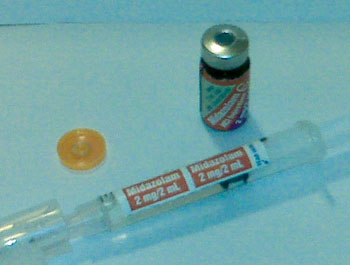 Figure 1. Top photo demonstrates medication vial with reusable adhesive medication label. This label is removed from the vial and applied to the syringe that was used to draw up the medication.
Figure 1. Top photo demonstrates medication vial with reusable adhesive medication label. This label is removed from the vial and applied to the syringe that was used to draw up the medication.
These cases had common characteristics. In every case the medication was drawn into an unlabeled syringe either with the intent of subsequently finding and applying the correct label, hand writing the label, or of never labeling the syringe at all but instead immediately administering the medication. A propagating factor in most was the absence of a proper pre-printed label immediately available for the medication at issue.
Complications from unlabeled or mislabeled syringes has in part prompted the Joint Commission on Accreditation of Healthcare Organizations to create National Patient Safety Goal 03.04.01: “Label all medications, medication containers (for example, syringes, medicine cups, basins), or other solutions on and off the sterile field.” Suggestions for compliance have included special label printers2,3 and computerized barcode systems.4 A common institutional remedy is to threaten disciplinary action against individual anesthesiologists for “not labeling your syringes” while simultaneously failing to make proper labels available. And the unfortunate selection by JCAHO of unlabeled propofol syringes as appropriate for administrative sanction5 reduces the issue to farce when in fact a real problem exists.
With the Medication Safety in the Operating Room Conference having been held in January, we would like to offer a simple, practical, and low cost approach that allows for compliance with the JCAHO standard, will likely reduce medication errors, and can be implemented using existing infrastructure in any hospital.
It is so simple it can be stated on only a few words: “A re-usable syringe-compatible label is applied (by the pharmacy or the manufacturer) over the cap of every injectable medication used in the operating room.” This solution both provides the right label for the right medication and also compels the anesthesiologist to remove the label to access the drug. The most obvious place to put the label is on the empty syringe that is about to be filled. This eliminates the error of “I filled the syringe but there was no label.” Roche has recently adapted this idea for some vials of midazolam as depicted in Figure 1.
Should the FDA require this type of packaging for all injectable medications? We think so. A standardization of the size, color, and font of labels for all injectable drugs, detachable from the top of the vial, would make proper syringe labeling a quick, uniform, effective, and safe process.
Benjamin O. Pate, BS
Medical Student IV
Bruce P. Kingsley, MD
Tucson, AZ
References
- Orser BA, et al. Medication errors in anesthetic practice: a survey of 687 practitioners. Can J Anesth 2001;48:139-46.
- Suyderhoud JP. Joint Commission on Accreditation of Healthcare Organizations requirements and syringe labeling systems. Anesth Analg 2007;104:242
- Foster P. Labeling history reviewed and future explored. APSF Newsletter 2006;20(4):86-7.
- Stabile M, et al. Medication administration in anesthesia: time for a paradigm shift. APSF Newsletter 2007;22(3):44-7.
- Allen G. Labeling syringes. APSF Newsletter 2007;22(3):54.
|
APSF Executive Committee Invites Collaboration From time to time the Anesthesia Patient Safety Foundation reconfirms its commitment of working with all who devote their energies to making anesthesia as safe as humanly possible. Thus, the Foundation invites collaboration from all who administer anesthesia, and all who provide the settings in which anesthesia is practiced, all individuals and all organizations who, through their work, affect the safety of patients receiving anesthesia. All will find us eager to listen to their suggestions and to work with them toward the common goal of safe anesthesia for all patients. |

 Figure 1. Top photo demonstrates medication vial with reusable adhesive medication label. This label is removed from the vial and applied to the syringe that was used to draw up the medication.
Figure 1. Top photo demonstrates medication vial with reusable adhesive medication label. This label is removed from the vial and applied to the syringe that was used to draw up the medication.

 Issue PDF
Issue PDF